PB1-F2 influenza A virus protein adopts a beta-sheet conformation and forms amyloid fibers in membrane environments
- PMID: 20172856
- PMCID: PMC2857135
- DOI: 10.1074/jbc.M109.067710
PB1-F2 influenza A virus protein adopts a beta-sheet conformation and forms amyloid fibers in membrane environments
Abstract
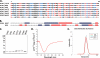
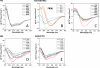
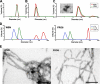
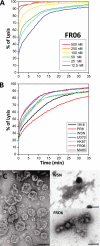
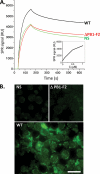
The influenza A virus PB1-F2 protein, encoded by an alternative reading frame in the PB1 polymerase gene, displays a high sequence polymorphism and is reported to contribute to viral pathogenesis in a sequence-specific manner. To gain insights into the functions of PB1-F2, the molecular structure of several PB1-F2 variants produced in Escherichia coli was investigated in different environments. Circular dichroism spectroscopy shows that all variants have a random coil secondary structure in aqueous solution. When incubated in trifluoroethanol polar solvent, all PB1-F2 variants adopt an alpha-helix-rich structure, whereas incubated in acetonitrile, a solvent of medium polarity mimicking the membrane environment, they display beta-sheet secondary structures. Incubated with asolectin liposomes and SDS micelles, PB1-F2 variants also acquire a beta-sheet structure. Dynamic light scattering revealed that the presence of beta-sheets is correlated with an oligomerization/aggregation of PB1-F2. Electron microscopy showed that PB1-F2 forms amorphous aggregates in acetonitrile. In contrast, at low concentrations of SDS, PB1-F2 variants exhibited various abilities to form fibers that were evidenced as amyloid fibers in a thioflavin T assay. Using a recombinant virus and its PB1-F2 knock-out mutant, we show that PB1-F2 also forms amyloid structures in infected cells. Functional membrane permeabilization assays revealed that the PB1-F2 variants can perforate membranes at nanomolar concentrations but with activities found to be sequence-dependent and not obviously correlated with their differential ability to form amyloid fibers. All of these observations suggest that PB1-F2 could be involved in physiological processes through different pathways, permeabilization of cellular membranes, and amyloid fiber formation.
Figures

References
-
- Lamb R. A., Takeda M. (2001) Nat. Med. 7, 1286–1288 - PubMed
-
- Gambotto A., Barratt-Boyes S. M., de Jong M. D., Neumann G., Kawaoka Y. (2008) Lancet 371, 1464–1475 - PubMed
-
- Garten R. J., Davis C. T., Russell C. A., Shu B., Lindstrom S., Balish A., Sessions W. M., Xu X., Skepner E., Deyde V., Okomo-Adhiambo M., Gubareva L., Barnes J., Smith C. B., Emery S. L., Hillman M. J., Rivailler P., Smagala J., de Graaf M., Burke D. F., Fouchier R. A., Pappas C., Alpuche-Aranda C. M., López-Gatell H., Olivera H., López I., Myers C. A., Faix D., Blair P. J., Yu C., Keene K. M., Dotson P. D., Jr., Boxrud D., Sambol A. R., Abid S. H., St George K., Bannerman T., Moore A. L., Stringer D. J., Blevins P., Demmler-Harrison G. J., Ginsberg M., Kriner P., Waterman S., Smole S., Guevara H. F., Belongia E. A., Clark P. A., Beatrice S. T., Donis R., Katz J., Finelli L., Bridges C. B., Shaw M., Jernigan D. B., Uyeki T. M., Smith D. J., Klimov A. I., Cox N. J. (2009) Science 325, 197–201 - PubMed
-
- Palese P. (1977) Cell 10, 1–10 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

