Female mice expressing constitutively active mutants of FSH receptor present with a phenotype of premature follicle depletion and estrogen excess
- PMID: 20172968
- PMCID: PMC2851188
- DOI: 10.1210/en.2009-0966
Female mice expressing constitutively active mutants of FSH receptor present with a phenotype of premature follicle depletion and estrogen excess
Abstract
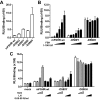
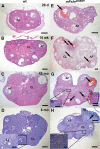
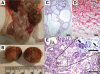
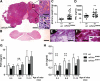
Strong gain-of-function mutations have not been identified in humans in the FSH receptor (FSHR), whereas such mutations are common among many other G protein-coupled receptors. In order to predict consequences of such mutations on humans, we first identified constitutively activated mutants of the mouse (m) Fshr and then expressed them under the human anti-Müllerian hormone promoter in transgenic mice or created knock-in mutation into the mouse genome. We show here that mutations of Asp580 in the mFSHR significantly increase the basal receptor activity. D580H and D580Y mutations of mFSHR bind FSH, but the activity of the former is neither ligand-dependent nor promiscuous towards LH/human choriogonadotropin stimulation. Transgenic expression of mFshr(D580H) in granulosa cells leads to abnormal ovarian structure and function in the form of hemorrhagic cysts, accelerated loss of small follicles, augmented granulosa cell proliferation, increased estradiol biosynthesis, and occasional luteinized unruptured follicles or teratomas. The most affected mFshr(D580H) females are infertile with disturbed estrous cycle and decreased gonadotropin and increased prolactin levels. Increased estradiol and prolactin apparently underlie the enhanced development of the mammary glands, adenomatous pituitary growth, and lipofuscin accumulation in the adrenal gland. The influence of the mFSHR(D580Y) mutation is milder, mainly causing hemorrhagic cysts in transgenic mFSHR(D580Y) and mFSHR(D580Y) -knock-in mice. The results demonstrate that gain-of-function mutations of the FSHR in mice bring about distinct and clear changes in ovarian function, informative in the search of similar mutations in humans.
Figures
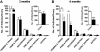
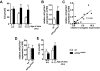
Similar articles
-
Molecular cloning of the mouse follicle-stimulating hormone receptor complementary deoxyribonucleic acid: functional expression of alternatively spliced variants and receptor inactivation by a C566T transition in exon 7 of the coding sequence.Biol Reprod. 1999 Jun;60(6):1515-27. doi: 10.1095/biolreprod60.6.1515. Biol Reprod. 1999. PMID: 10330114
-
Normalization of hormonal imbalances, ovarian follicular dynamics and metabolic effects in follitrophin receptor knockout mice.Mol Hum Reprod. 2007 May;13(5):287-97. doi: 10.1093/molehr/gam008. Epub 2007 Mar 9. Mol Hum Reprod. 2007. PMID: 17350962
-
Targeted disruption of the estrogen receptor-alpha gene in female mice: characterization of ovarian responses and phenotype in the adult.Endocrinology. 1999 Jun;140(6):2733-44. doi: 10.1210/endo.140.6.6823. Endocrinology. 1999. PMID: 10342864
-
Mutations in the follicle-stimulating hormone-beta (FSH beta) and FSH receptor genes in mice and humans.Semin Reprod Med. 2000;18(1):5-10. doi: 10.1055/s-2000-13470. Semin Reprod Med. 2000. PMID: 11299519 Review.
-
Mutations and polymorphisms of the FSH receptor (FSHR) gene: clinical implications in female fecundity and molecular biology of FSHR protein and gene.Obstet Gynecol Surv. 2008 Dec;63(12):785-95. doi: 10.1097/OGX.0b013e31818957eb. Obstet Gynecol Surv. 2008. PMID: 19017414 Review.
Cited by
-
Animal models for aberrations of gonadotropin action.Rev Endocr Metab Disord. 2011 Dec;12(4):245-58. doi: 10.1007/s11154-011-9174-4. Rev Endocr Metab Disord. 2011. PMID: 21484328 Free PMC article. Review.
-
Infertility in Female Mice with a Gain-of-Function Mutation in the Luteinizing Hormone Receptor Is Due to Irregular Estrous Cyclicity, Anovulation, Hormonal Alterations, and Polycystic Ovaries.Biol Reprod. 2015 Jul;93(1):16. doi: 10.1095/biolreprod.115.129072. Epub 2015 Jun 3. Biol Reprod. 2015. PMID: 26040673 Free PMC article.
-
Gain-of-Function Genetic Models to Study FSH Action.Front Endocrinol (Lausanne). 2019 Feb 7;10:28. doi: 10.3389/fendo.2019.00028. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 30792692 Free PMC article. Review.
-
Role of the inositol polyphosphate-4-phosphatase type II Inpp4b in the generation of ovarian teratomas.Dev Biol. 2013 Jan 1;373(1):118-29. doi: 10.1016/j.ydbio.2012.10.011. Epub 2012 Oct 16. Dev Biol. 2013. PMID: 23078915 Free PMC article.
-
The Roles of Luteinizing Hormone, Follicle-Stimulating Hormone and Testosterone in Spermatogenesis and Folliculogenesis Revisited.Int J Mol Sci. 2021 Nov 25;22(23):12735. doi: 10.3390/ijms222312735. Int J Mol Sci. 2021. PMID: 34884539 Free PMC article. Review.
References
-
- Aittomäki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehväslaiho H, Engel AR, Nieschlag E, Huhtaniemi I, de la Chapelle A 1995 Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell 82:959–968 - PubMed
-
- Themmen APN, Huhtaniemi IT 2000 Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev 21:551–583 - PubMed
-
- Matthews CH, Borgato S, Beck-Peccoz P, Adams M, Tone Y, Gambino G, Casagrande S, Tedeschini G, Benedetti A, Chatterjee VK 1993 Primary amenorrhoea and infertility due to a mutation in the β-subunit of follicle-stimulating hormone. Nat Genet 5:83–86 - PubMed
-
- Oktay K, Briggs D, Gosden RG 1997 Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab 82:3748–3751 - PubMed
-
- Rannikki AS, Zhang FP, Huhtaniemi IT 1995 Ontogeny of follicle-stimulating hormone receptor gene expression in the rat testis and ovary. Mol Cell Endocrinol 107:199–208 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

