Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure
- PMID: 20173042
- PMCID: PMC2867447
- DOI: 10.1152/ajpheart.01006.2009
Chronic AT1 receptor blockade normalizes NMDA-mediated changes in renal sympathetic nerve activity and NR1 expression within the PVN in rats with heart failure
Abstract
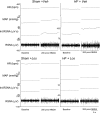
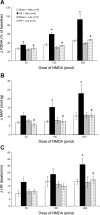
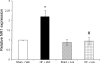
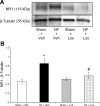
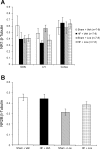
Exercise training normalizes enhanced glutamatergic mechanisms within the paraventricular nucleus (PVN) concomitant with the normalization of increased plasma ANG II levels in rats with heart failure (HF). We tested whether ANG II type 1 (AT(1)) receptors are involved in the normalization of PVN glutamatergic mechanisms using chronic AT(1) receptor blockade with losartan (Los; 50 mg.kg(-1).day(-1) in drinking water for 3 wk). Left ventricular end-diastolic pressure was increased in both HF + vehicle (Veh) and HF + Los groups compared with sham-operated animals (Sham group), although it was significantly attenuated in the HF + Los group compared with the HF + Veh group. The effect of Los on cardiac function was similar to exercise training. At the highest dose of N-methyl-d-aspartate (NMDA; 200 pmol) injected into the PVN, the increase in renal sympathetic nerve activity was 93 +/- 13% in the HF + Veh group, which was significantly higher (P < 0.05) than the increase in the Sham + Veh (45 +/- 2%) and HF + Los (47 +/- 2%) groups. Relative NMDA receptor subunit NR(1) mRNA expression within the PVN was increased 120% in the HF + Veh group compared with the Sham + Veh group (P < 0.05) but was significantly attenuated in the HF + Los group compared with the HF + Veh group (P < 0.05). NR(1) protein expression increased 87% in the HF + Veh group compared with the Sham + Veh group but was significantly attenuated in the HF + Los group compared with the HF + Veh group (P < 0.05). Furthermore, in in vitro experiments using neuronal NG-108 cells, we found that ANG II treatment stimulated NR(1) protein expression and that Los significantly ameliorated the NR(1) expression induced by ANG II. These data are consistent with our hypothesis that chronic AT(1) receptor blockade normalizes glutamatergic mechanisms within the PVN in rats with HF.
Figures


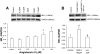
Similar articles
-
Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure.Circ Res. 2003 Nov 14;93(10):990-7. doi: 10.1161/01.RES.0000102865.60437.55. Epub 2003 Oct 23. Circ Res. 2003. PMID: 14576197
-
Exercise training normalizes enhanced glutamate-mediated sympathetic activation from the PVN in heart failure.Am J Physiol Regul Integr Comp Physiol. 2008 Jun;294(6):R1863-72. doi: 10.1152/ajpregu.00757.2007. Epub 2008 Apr 2. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18385465 Free PMC article.
-
Exercise training normalizes enhanced sympathetic activation from the paraventricular nucleus in chronic heart failure: role of angiotensin II.Am J Physiol Regul Integr Comp Physiol. 2012 Aug 15;303(4):R387-94. doi: 10.1152/ajpregu.00046.2012. Epub 2012 Jun 20. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22718804 Free PMC article.
-
Central neural control of sympathetic nerve activity in heart failure following exercise training.Am J Physiol Heart Circ Physiol. 2012 Feb 1;302(3):H527-37. doi: 10.1152/ajpheart.00676.2011. Epub 2011 Nov 18. Am J Physiol Heart Circ Physiol. 2012. PMID: 22101524 Free PMC article. Review.
-
Role of the hypothalamic PVN in the regulation of renal sympathetic nerve activity and blood flow during hyperthermia and in heart failure.Am J Physiol Renal Physiol. 2010 Apr;298(4):F839-46. doi: 10.1152/ajprenal.00734.2009. Epub 2010 Feb 10. Am J Physiol Renal Physiol. 2010. PMID: 20147365 Free PMC article. Review.
Cited by
-
Exercise training augments neuronal nitric oxide synthase dimerization in the paraventricular nucleus of rats with chronic heart failure.Nitric Oxide. 2019 Jun 1;87:73-82. doi: 10.1016/j.niox.2019.03.005. Epub 2019 Mar 13. Nitric Oxide. 2019. PMID: 30878404 Free PMC article.
-
Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-α.Hypertension. 2014 Mar;63(3):527-34. doi: 10.1161/HYPERTENSIONAHA.113.02429. Epub 2013 Dec 9. Hypertension. 2014. PMID: 24324037 Free PMC article.
-
Overexpression of angiotensin-converting enzyme 2 attenuates tonically active glutamatergic input to the rostral ventrolateral medulla in hypertensive rats.Am J Physiol Heart Circ Physiol. 2014 Jul 15;307(2):H182-90. doi: 10.1152/ajpheart.00518.2013. Epub 2014 May 16. Am J Physiol Heart Circ Physiol. 2014. PMID: 24838502 Free PMC article.
-
Centrally mediated erectile dysfunction in rats with type 1 diabetes: role of angiotensin II and superoxide.J Sex Med. 2013 Sep;10(9):2165-76. doi: 10.1111/jsm.12248. Epub 2013 Jul 10. J Sex Med. 2013. PMID: 23841890 Free PMC article.
-
Effect of Prolonged Moderate Exercise on the Changes of Nonneuronal Cells in Early Myocardial Infarction.Neural Plast. 2015;2015:265967. doi: 10.1155/2015/265967. Epub 2015 Jul 22. Neural Plast. 2015. PMID: 26266053 Free PMC article.
References
-
- Allen AM, Dampney RA, Mendelsohn FA. Angiotensin receptor binding and pressor effects in cat subretrofacial nucleus. Am J Physiol Heart Circ Physiol 255: H1011–H1017, 1988 - PubMed
-
- Anderson JW, Smith PM, Ferguson AV. Subfornical organ neurons projecting to paraventricular nucleus: whole-cell properties. Brain Res 921: 78–85, 2001 - PubMed
-
- Bui JD, Kimura B, Phillips MI. Losartan potassium, a nonpeptide antagonist of angiotensin II, chronically administered p.o. does not readily cross the blood-brain barrier. Eur J Pharmacol 219: 147–151, 1992 - PubMed
-
- Bunnemann B, Fuxe K, Ganten D. The renin-angiotensin system in the brain: an update 1993. Regul Pept 46: 487–509, 1993 - PubMed
-
- Casto R, Phillips MI. Cardiovascular actions of microinjections of angiotensin II in the brain stem of rats. Am J Physiol Regul Integr Comp Physiol 246: R811–R816, 1984 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

