Impact of endochitinase-transformed white spruce on soil fungal biomass and ectendomycorrhizal symbiosis
- PMID: 20173071
- PMCID: PMC2849194
- DOI: 10.1128/AEM.02807-09
Impact of endochitinase-transformed white spruce on soil fungal biomass and ectendomycorrhizal symbiosis
Abstract
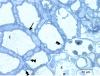
The impact of transgenic white spruce [Picea glauca (Moench) Voss] containing the endochitinase gene (ech42) on soil fungal biomass and on the ectendomycorrhizal fungi Wilcoxina spp. was tested using a greenhouse trial. The measured level of endochitinase in roots of transgenic white spruce was up to 10 times higher than that in roots of nontransformed white spruce. The level of endochitinase in root exudates of three of four ech42-transformed lines was significantly greater than that in controls. Analysis soil ergosterol showed that the amount of fungal biomass in soil samples from control white spruce was slightly larger than that in soil samples from ech42-transformed white spruce. Nevertheless, the difference was not statistically significant. The rates of mycorrhizal colonization of transformed lines and controls were similar. Sequencing the internal transcribed spacer rRNA region revealed that the root tips were colonized by the ectendomycorrhizal fungi Wilcoxina spp. and the dark septate endophyte Phialocephala fortinii. Colonization of root tips by Wilcoxina spp. was monitored by real-time PCR to quantify the fungus present during the development of ectendomycorrhizal symbiosis in ech42-transformed and control lines. The numbers of Wilcoxina molecules in the transformed lines and the controls were not significantly different (P > 0.05, as determined by analysis of covariance), indicating that in spite of higher levels of endochitinase expression, mycorrhization was not inhibited. Our results indicate that the higher levels of chitinolytic activity in root exudates and root tissues from ech42-transformed lines did not alter the soil fungal biomass or the development of ectendomycorrhizal symbiosis involving Wilcoxina spp.
Figures
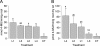
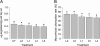


Similar articles
-
Impact of endochitinase-transformed white spruce on soil fungal communities under greenhouse conditions.FEMS Microbiol Ecol. 2011 May;76(2):199-208. doi: 10.1111/j.1574-6941.2011.01041.x. Epub 2011 Jan 26. FEMS Microbiol Ecol. 2011. PMID: 21223334
-
Genetic host-tree effects on the ectomycorrhizal community and root characteristics of Norway spruce.Mycorrhiza. 2013 Jan;23(1):21-33. doi: 10.1007/s00572-012-0446-y. Epub 2012 May 30. Mycorrhiza. 2013. PMID: 22644394
-
Long-term effect of apatite on ectomycorrhizal growth and community structure.Mycorrhiza. 2012 Nov;22(8):615-21. doi: 10.1007/s00572-012-0438-y. Epub 2012 Mar 27. Mycorrhiza. 2012. PMID: 22451218
-
Mycorrhiza reduces adverse effects of dark septate endophytes (DSE) on growth of conifers.PLoS One. 2012;7(8):e42865. doi: 10.1371/journal.pone.0042865. Epub 2012 Aug 10. PLoS One. 2012. PMID: 22900058 Free PMC article.
-
Root-associated ectomycorrhizal fungi shared by various boreal forest seedlings naturally regenerating after a fire in interior alaska and correlation of different fungi with host growth responses.Appl Environ Microbiol. 2011 May;77(10):3351-9. doi: 10.1128/AEM.02575-10. Epub 2011 Mar 25. Appl Environ Microbiol. 2011. PMID: 21441343 Free PMC article.
Cited by
-
Chitinase activities, scab resistance, mycorrhization rates and biomass of own-rooted and grafted transgenic apple.Genet Mol Biol. 2012 Apr;35(2):466-73. doi: 10.1590/S1415-47572012000300014. Epub 2012 Jun 23. Genet Mol Biol. 2012. PMID: 22888297 Free PMC article.
-
The expression pattern of the Picea glauca Defensin 1 promoter is maintained in Arabidopsis thaliana, indicating the conservation of signalling pathways between angiosperms and gymnosperms.J Exp Bot. 2012 Jan;63(2):785-95. doi: 10.1093/jxb/err303. Epub 2011 Nov 2. J Exp Bot. 2012. PMID: 22048038 Free PMC article.
-
Emerging from the ice-fungal communities are diverse and dynamic in earliest soil developmental stages of a receding glacier.Environ Microbiol. 2019 May;21(5):1864-1880. doi: 10.1111/1462-2920.14598. Epub 2019 Apr 11. Environ Microbiol. 2019. PMID: 30888722 Free PMC article.
-
The effects of high-tannin leaf litter from transgenic poplars on microbial communities in microcosm soils.Front Microbiol. 2013 Sep 26;4:290. doi: 10.3389/fmicb.2013.00290. eCollection 2013. Front Microbiol. 2013. PMID: 24133486 Free PMC article.
-
Assessing Impacts of Transgenic Plants on Soil Using Functional Indicators: Twenty Years of Research and Perspectives.Plants (Basel). 2022 Sep 19;11(18):2439. doi: 10.3390/plants11182439. Plants (Basel). 2022. PMID: 36145839 Free PMC article. Review.
References
-
- Addy, H. D., M. M. Piercey, and R. S. Currah. 2005. Microfungal endophytes in roots. Can. J. Bot. 83:1-13.
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Blackwell, J. 1988. Physical methods for the determination of chitin structure and conformation. Methods Enzymol. 161:435-442.
-
- Bolar, J. P., J. L. Norelli, K.-W. Wong, C. K. Hayes, G. E. Harman, and H. S. Aldwinckle. 2000. Expression of endochitinase from Trichoderma harzianum in transgenic apple increases resistance to apple scab and reduces vigor. Phytopathology 90:72-77. - PubMed
-
- Bolar, J. P., J. L. Norelli, G. E. Harman, S. K. Brown, and H. S. Aldwinckle. 2001. Synergistic activity of endochitinase and exochitinase from Trichoderma atroviride (T. harzianum) against the pathogenic fungus (Venturia inaequalis) in transgenic apple plants. Transgenic Res. 10:533-543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

