Persistent Nav1.6 current at axon initial segments tunes spike timing of cerebellar granule cells
- PMID: 20173079
- PMCID: PMC2828138
- DOI: 10.1113/jphysiol.2010.183798
Persistent Nav1.6 current at axon initial segments tunes spike timing of cerebellar granule cells
Erratum in
- J Physiol. 2011 Jan 15;589(Pt 2):447
Abstract
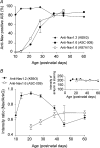
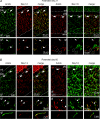
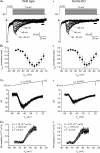
Cerebellar granule (CG) cells generate high-frequency action potentials that have been proposed to depend on the unique properties of their voltage-gated ion channels. To address the in vivo function of Nav1.6 channels in developing and mature CG cells, we combined the study of the developmental expression of Nav subunits with recording of acute cerebellar slices from young and adult granule-specific Scn8a KO mice. Nav1.2 accumulated rapidly at early-formed axon initial segments (AISs). In contrast, Nav1.6 was absent at early postnatal stages but accumulated at AISs of CG cells from P21 to P40. By P40-P65, both Nav1.6 and Nav1.2 co-localized at CG cell AISs. By comparing Na(+) currents in mature CG cells (P66-P74) from wild-type and CG-specific Scn8a KO mice, we found that transient and resurgent Na(+) currents were not modified in the absence of Nav1.6 whereas persistent Na(+) current was strongly reduced. Action potentials in conditional Scn8a KO CG cells showed no alteration in threshold and overshoot, but had a faster repolarization phase and larger post-spike hyperpolarization. In addition, although Scn8a KO CG cells kept their ability to fire action potentials at very high frequency, they displayed increased interspike-interval variability and firing irregularity in response to sustained depolarization. We conclude that Nav1.6 channels at axon initial segments contribute to persistent Na(+) current and ensure a high degree of temporal precision in repetitive firing of CG cells.
Figures




Similar articles
-
Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (NaV1.6) in cerebellar purkinje neurons and granule cells.J Neurophysiol. 2006 Aug;96(2):785-93. doi: 10.1152/jn.01193.2005. Epub 2006 May 10. J Neurophysiol. 2006. PMID: 16687615
-
Role of axonal NaV1.6 sodium channels in action potential initiation of CA1 pyramidal neurons.J Neurophysiol. 2008 Oct;100(4):2361-80. doi: 10.1152/jn.90332.2008. Epub 2008 Jul 23. J Neurophysiol. 2008. PMID: 18650312
-
The absence of resurgent sodium current in mouse spinal neurons.Brain Res. 1999 Dec 4;849(1-2):162-8. doi: 10.1016/s0006-8993(99)02060-0. Brain Res. 1999. PMID: 10592298
-
Cerebellum-related characteristics of Scn8a-mutant mice.Cerebellum. 2009 Sep;8(3):192-201. doi: 10.1007/s12311-009-0110-z. Epub 2009 May 8. Cerebellum. 2009. PMID: 19424768 Review.
-
Molecular diversity of structure and function of the voltage-gated Na+ channels.Jpn J Pharmacol. 2002 Apr;88(4):365-77. doi: 10.1254/jjp.88.365. Jpn J Pharmacol. 2002. PMID: 12046980 Review.
Cited by
-
Differential Inhibition of Human Nav1.2 Resurgent and Persistent Sodium Currents by Cannabidiol and GS967.Int J Mol Sci. 2020 Apr 1;21(7):2454. doi: 10.3390/ijms21072454. Int J Mol Sci. 2020. PMID: 32244818 Free PMC article.
-
Components of action potential repolarization in cerebellar parallel fibres.J Physiol. 2014 Nov 15;592(22):4911-29. doi: 10.1113/jphysiol.2014.280719. Epub 2014 Sep 19. J Physiol. 2014. PMID: 25239461 Free PMC article.
-
The effects of the general anesthetic sevoflurane on neurotransmission: an experimental and computational study.Sci Rep. 2021 Feb 22;11(1):4335. doi: 10.1038/s41598-021-83714-y. Sci Rep. 2021. PMID: 33619298 Free PMC article.
-
De novo gain-of-function and loss-of-function mutations of SCN8A in patients with intellectual disabilities and epilepsy.J Med Genet. 2015 May;52(5):330-7. doi: 10.1136/jmedgenet-2014-102813. Epub 2015 Feb 27. J Med Genet. 2015. PMID: 25725044 Free PMC article.
-
SCN8A encephalopathy: Research progress and prospects.Epilepsia. 2016 Jul;57(7):1027-35. doi: 10.1111/epi.13422. Epub 2016 Jun 8. Epilepsia. 2016. PMID: 27270488 Free PMC article.
References
-
- Afshari FS, Ptak K, Khaliq ZM, Grieco TM, Slater NT, McCrimmon DR, Raman IM. Resurgent Na currents in four classes of neurons of the cerebellum. J Neurophysiol. 2004;92:2831–2843. - PubMed
-
- Aller MI, Jones A, Merlo D, Paterlini M, Meyer AH, Amtmann U, Brickley S, Jolin HE, McKenzie AN, Monyer H, Farrant M, Wisden W. Cerebellar granule cell Cre recombinase expression. Genesis. 2003;36:97–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

