Alternative modes of GM-CSF receptor activation revealed using activated mutants of the common beta-subunit
- PMID: 20173116
- PMCID: PMC2858480
- DOI: 10.1182/blood-2009-08-235846
Alternative modes of GM-CSF receptor activation revealed using activated mutants of the common beta-subunit
Abstract
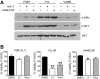
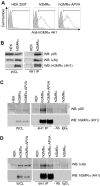
Granulocyte/macrophage colony-stimulating factor promotes growth, survival, differentiation, and activation of normal myeloid cells and plays an important role in myeloid leukemias. The GM-CSF receptor (GMR) shares a signaling subunit, beta(c), with interleukin-3 and interleukin-5 receptors and has recently been shown to induce activation of Janus kinase 2 (JAK2) and downstream signaling via formation of a unique dodecameric receptor complex. In this study we use 2 activated beta(c) mutants that display distinct signaling capacity and have differential requirements for the GMR alpha-subunit (GMR-alpha) to dissect the signaling pathways associated with the GM-CSF response. The V449E transmembrane mutant selectively activates JAK2/signal transducer and activator of transcription 5 and extracellular signal-regulated kinase (ERK) pathways, resulting in a high level of sensitivity to JAK and ERK inhibitors, whereas the extracellular mutant (FIDelta) selectively activates the phosphoinositide 3-kinase/Akt and IkappaKbeta/nuclear factorkappaB pathways. We also demonstrate a novel and direct interaction between the SH3 domains of Lyn and Src with a conserved proline-rich motif in GMR-alpha and show a selective requirement for Src family kinases by the FIDelta mutant. We relate the nonoverlapping nature of signaling by the activated mutants to the structure of the unique GMR complex and propose alternative modes of receptor activation acting synergistically in the mature liganded receptor complex.
Figures
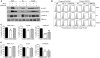

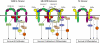
Similar articles
-
Tyrosine phosphorylation of Shc is not required for proliferation or viability signaling by granulocyte-macrophage colony-stimulating factor in hematopoietic cell lines.J Immunol. 1996 Jul 15;157(2):534-40. J Immunol. 1996. PMID: 8752899
-
Functional analysis of a single chain chimeric alpha/beta-granulocyte-macrophage colony-stimulating factor receptor. Importance of a glutamate residue in the transmembrane region.J Biol Chem. 1999 Nov 12;274(46):33064-71. doi: 10.1074/jbc.274.46.33064. J Biol Chem. 1999. PMID: 10551876
-
Signaling domains of the beta c chain of the GM-CSF/IL-3/IL-5 receptor.Ann N Y Acad Sci. 1999 Apr 30;872:305-12; discussion 312-3. doi: 10.1111/j.1749-6632.1999.tb08474.x. Ann N Y Acad Sci. 1999. PMID: 10372132 Review.
-
Evidence for a physical association between the Shc-PTB domain and the beta c chain of the granulocyte-macrophage colony-stimulating factor receptor.J Biol Chem. 1996 May 24;271(21):12137-40. doi: 10.1074/jbc.271.21.12137. J Biol Chem. 1996. PMID: 8647804
-
Roles of JAK kinases in human GM-CSF receptor signal transduction.J Allergy Clin Immunol. 1996 Dec;98(6 Pt 2):S183-91. doi: 10.1016/s0091-6749(96)70065-9. J Allergy Clin Immunol. 1996. PMID: 8977526 Review.
Cited by
-
Dual Role of GM-CSF as a Pro-Inflammatory and a Regulatory Cytokine: Implications for Immune Therapy.J Interferon Cytokine Res. 2015 Aug;35(8):585-99. doi: 10.1089/jir.2014.0149. Epub 2015 Mar 24. J Interferon Cytokine Res. 2015. PMID: 25803788 Free PMC article. Review.
-
Protein kinase activity of phosphoinositide 3-kinase regulates cytokine-dependent cell survival.PLoS Biol. 2013;11(3):e1001515. doi: 10.1371/journal.pbio.1001515. Epub 2013 Mar 19. PLoS Biol. 2013. PMID: 23526884 Free PMC article.
-
Screening of the FDA-approved drug library identifies CCL17 inhibitors that block arthritic pain.Sci Rep. 2025 Jul 23;15(1):26734. doi: 10.1038/s41598-025-12191-4. Sci Rep. 2025. PMID: 40702243 Free PMC article.
-
NF-κB inhibitors impair lung epithelial tight junctions in the absence of inflammation.Tissue Barriers. 2015 Apr 3;3(1-2):e982424. doi: 10.4161/21688370.2014.982424. eCollection 2015. Tissue Barriers. 2015. PMID: 25838984 Free PMC article.
-
Role of the β Common (βc) Family of Cytokines in Health and Disease.Cold Spring Harb Perspect Biol. 2018 Jun 1;10(6):a028514. doi: 10.1101/cshperspect.a028514. Cold Spring Harb Perspect Biol. 2018. PMID: 28716883 Free PMC article. Review.
References
-
- Kondo M, Scherer DC, Miyamoto T, et al. Cell-fate conversion of lymphoid-committed progenitors by instructive actions of cytokines. Nature. 2000;407(6802):383–386. - PubMed
-
- Conti L, Gessani S. GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology. 2008;213(9-10):859–870. - PubMed
-
- Ikegami M, Ueda T, Hull W, et al. Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol. 1996;270(4 Pt 1):650–658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

