Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys
- PMID: 20173752
- PMCID: PMC2834868
- DOI: 10.1038/nm.2089
Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys
Abstract
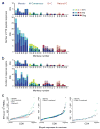
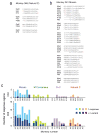
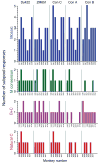
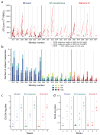
The worldwide diversity of HIV-1 presents an unprecedented challenge for vaccine development. Antigens derived from natural HIV-1 sequences have elicited only a limited breadth of cellular immune responses in nonhuman primate studies and clinical trials to date. Polyvalent 'mosaic' antigens, in contrast, are designed to optimize cellular immunologic coverage of global HIV-1 sequence diversity. Here we show that mosaic HIV-1 Gag, Pol and Env antigens expressed by recombinant, replication-incompetent adenovirus serotype 26 vectors markedly augmented both the breadth and depth without compromising the magnitude of antigen-specific T lymphocyte responses as compared with consensus or natural sequence HIV-1 antigens in rhesus monkeys. Polyvalent mosaic antigens therefore represent a promising strategy to expand cellular immunologic vaccine coverage for genetically diverse pathogens such as HIV-1.
Conflict of interest statement
Figures
Comment in
-
HIV vaccines: mosaic approach to virus diversity.Nat Med. 2010 Mar;16(3):268-70. doi: 10.1038/nm0310-268. Nat Med. 2010. PMID: 20208511 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- U19 AI078526/AI/NIAID NIH HHS/United States
- U01 AI067854/AI/NIAID NIH HHS/United States
- AI061734/AI/NIAID NIH HHS/United States
- P01 AI061734/AI/NIAID NIH HHS/United States
- R01 AI066924/AI/NIAID NIH HHS/United States
- AI078526/AI/NIAID NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
- U19 AI066305/AI/NIAID NIH HHS/United States
- AI066305/AI/NIAID NIH HHS/United States
- RR000168/RR/NCRR NIH HHS/United States
- P51 RR000168/RR/NCRR NIH HHS/United States
- AI084794/AI/NIAID NIH HHS/United States
- K26 RR000168/RR/NCRR NIH HHS/United States
- R01 AI084794/AI/NIAID NIH HHS/United States
- AI067854/AI/NIAID NIH HHS/United States
- AI066924/AI/NIAID NIH HHS/United States
- U19 AI067854/AI/NIAID NIH HHS/United States
- AI058727/AI/NIAID NIH HHS/United States
- R01 AI058727/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

