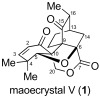
Synthetic Studies towards Maoecrystal V
- PMID: 20174449
- PMCID: PMC2822274
- DOI: 10.1016/j.tetlet.2009.09.050
Synthetic Studies towards Maoecrystal V
Abstract
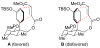
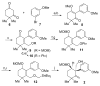
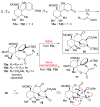
The investigation of an intramolecular Diels-Alder reaction, en route to a projected total synthesis of maoecrystal V, is described.
Figures
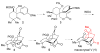
Similar articles
-
Toward the total synthesis of maoecrystal V: an intramolecular Diels-Alder route to the maoecrystal V pentacyclic core with the appropriate relative stereochemistry.Tetrahedron Lett. 2011 Apr 27;52(17):2104-2106. doi: 10.1016/j.tetlet.2010.11.029. Tetrahedron Lett. 2011. PMID: 21516211 Free PMC article.
-
Total synthesis of maoecrystal V.Chem Asian J. 2015 Apr;10(4):903-9. doi: 10.1002/asia.201403228. Epub 2014 Dec 12. Chem Asian J. 2015. PMID: 25504983
-
Enantioselective synthesis of (-)-maoecrystal V by enantiodetermining C-H functionalization.J Am Chem Soc. 2014 Dec 24;136(51):17738-49. doi: 10.1021/ja510573v. Epub 2014 Dec 11. J Am Chem Soc. 2014. PMID: 25409033 Free PMC article.
-
Application of the aza-Diels-Alder reaction in the synthesis of natural products.Org Biomol Chem. 2017 Apr 11;15(15):3105-3129. doi: 10.1039/c6ob02761j. Org Biomol Chem. 2017. PMID: 28327756 Review.
-
Recent advancements in the chemistry of Diels-Alder reaction for total synthesis of natural products: a comprehensive review (2020-2023).RSC Adv. 2025 Feb 10;15(6):4496-4525. doi: 10.1039/d4ra07989b. eCollection 2025 Feb 6. RSC Adv. 2025. PMID: 39931410 Free PMC article. Review.
Cited by
-
Dearomative logic in natural product total synthesis.Nat Prod Rep. 2022 Dec 14;39(12):2231-2291. doi: 10.1039/d2np00042c. Nat Prod Rep. 2022. PMID: 36173020 Free PMC article. Review.
-
A synthesis of the carbon skeleton of maoecrystal V.Org Lett. 2009 Nov 5;11(21):4774-6. doi: 10.1021/ol901963v. Org Lett. 2009. PMID: 19795876 Free PMC article.
-
11-Step Total Synthesis of (-)-Maoecrystal V.J Am Chem Soc. 2016 Aug 3;138(30):9425-8. doi: 10.1021/jacs.6b06623. Epub 2016 Jul 26. J Am Chem Soc. 2016. PMID: 27457680 Free PMC article.
-
Total synthesis of maoecrystal V: early-stage C-H functionalization and lactone assembly by radical cyclization.J Am Chem Soc. 2013 Oct 2;135(39):14552-5. doi: 10.1021/ja408231t. Epub 2013 Sep 23. J Am Chem Soc. 2013. PMID: 24047444 Free PMC article.
-
Total synthesis of (±)-maoecrystal V.J Am Chem Soc. 2012 Nov 14;134(45):18860-7. doi: 10.1021/ja309905j. Epub 2012 Nov 5. J Am Chem Soc. 2012. PMID: 23126440 Free PMC article.
References
-
- Li S, Wang J, Niu X, Shen Y, Zhang H, Sun H, Li M, Tian Q, Lu Y, Cao P, Zheng Q. Org Lett. 2004;6:4327–4330. - PubMed
-
- Wilson RM, Danishefsky SJ. J Org Chem. 2007;72:4293–4305. - PubMed
-
- Fox JM, Huang X, Chieffi A, Buchwald S. J Am Chem Soc. 2000;122:1360–1370.
-
- Stork G, Danheiser R. J Org Chem. 1973;38:1775.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
