Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy
- PMID: 20174468
- PMCID: PMC2824828
- DOI: 10.1371/journal.pone.0009313
Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy
Retraction in
-
Retraction: Selective Molecular Alterations in the Autophagy Pathway in Patients with Lewy Body Disease and in Models of α-Synucleinopathy.PLoS One. 2024 Nov 13;19(11):e0313935. doi: 10.1371/journal.pone.0313935. eCollection 2024. PLoS One. 2024. PMID: 39536070 Free PMC article. No abstract available.
Abstract
Background: Lewy body disease is a heterogeneous group of neurodegenerative disorders characterized by alpha-synuclein accumulation that includes dementia with Lewy bodies (DLB) and Parkinson's Disease (PD). Recent evidence suggests that impairment of lysosomal pathways (i.e. autophagy) involved in alpha-synuclein clearance might play an important role. For this reason, we sought to examine the expression levels of members of the autophagy pathway in brains of patients with DLB and Alzheimer's Disease (AD) and in alpha-synuclein transgenic mice.
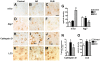
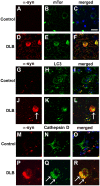
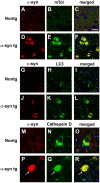
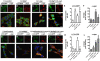
Methodology/principal findings: By immunoblot analysis, compared to controls and AD, in DLB cases levels of mTor were elevated and Atg7 were reduced. Levels of other components of the autophagy pathway such as Atg5, Atg10, Atg12 and Beclin-1 were not different in DLB compared to controls. In DLB brains, mTor was more abundant in neurons displaying alpha-synuclein accumulation. These neurons also showed abnormal expression of lysosomal markers such as LC3, and ultrastructural analysis revealed the presence of abundant and abnormal autophagosomes. Similar alterations were observed in the brains of alpha-synuclein transgenic mice. Intra-cerebral infusion of rapamycin, an inhibitor of mTor, or injection of a lentiviral vector expressing Atg7 resulted in reduced accumulation of alpha-synuclein in transgenic mice and amelioration of associated neurodegenerative alterations.
Conclusions/significance: This study supports the notion that defects in the autophagy pathway and more specifically in mTor and Atg7 are associated with neurodegeneration in DLB cases and alpha-synuclein transgenic models and supports the possibility that modulators of the autophagy pathway might have potential therapeutic effects.
Conflict of interest statement
Figures


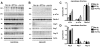
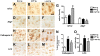


References
-
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39:889–909. - PubMed
-
- Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept Lett. 2004;11:213–228. - PubMed
-
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. - PubMed
-
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer's and Parkinson's diseases. Neuromolecular Med. 2003;4:21–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

