In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide
- PMID: 20174579
- PMCID: PMC2824823
- DOI: 10.1371/journal.pone.0009310
In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide
Abstract
Background: Tenofovir gel has entered into clinical trials for use as a topical microbicide to prevent HIV-1 infection but has no published data regarding pre-clinical testing using in vitro and ex vivo models. To validate our findings with on-going clinical trial results, we evaluated topical tenofovir gel for safety and efficacy. We also modeled systemic application of tenofovir for efficacy.
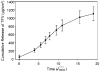
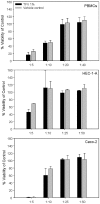
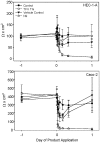
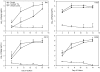
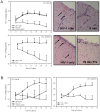
Methods and findings: Formulation assessment of tenofovir gel included osmolality, viscosity, in vitro release, and permeability testing. Safety was evaluated by measuring the effect on the viability of vaginal flora, PBMCs, epithelial cells, and ectocervical and colorectal explant tissues. For efficacy testing, PBMCs were cultured with tenofovir or vehicle control gels and HIV-1 representing subtypes A, B, and C. Additionally, polarized ectocervical and colorectal explant cultures were treated apically with either gel. Tenofovir was added basolaterally to simulate systemic application. All tissues were challenged with HIV-1 applied apically. Infection was assessed by measuring p24 by ELISA on collected supernatants and immunohistochemistry for ectocervical explants. Formulation testing showed the tenofovir and vehicle control gels were >10 times isosmolar. Permeability through ectocervical tissue was variable but in all cases the receptor compartment drug concentration reached levels that inhibit HIV-1 infection in vitro. The gels were non-toxic toward vaginal flora, PBMCs, or epithelial cells. A transient reduction in epithelial monolayer integrity and epithelial fracture for ectocervical and colorectal explants was noted and likely due to the hyperosmolar nature of the formulation. Tenofovir gel prevented HIV-1 infection of PBMCs regardless of HIV-1 subtype. Topical and systemic tenofovir were effective at preventing HIV-1 infection of explant cultures.
Conclusions: These studies provide a mechanism for pre-clinical prediction of safety and efficacy of formulated microbicides. Tenofovir was effective against HIV-1 infection in our algorithm. These data support the use of tenofovir for pre-exposure prophylaxis.
Conflict of interest statement
Figures



References
-
- UNAIDS/WHO. Geneva: UNAIDS.; 2008. Report on global AIDS epidemic.362
-
- Gray RH, Wawer MJ, Polis CB, Kigozi G, Serwadda D. Male Circumcision and Prevention of HIV and Sexually Transmitted Infections. Curr Infect Dis Rep. 2008;10:121–127. - PubMed
-
- Mills E, Cooper C, Anema A, Guyatt G. Male circumcision for the prevention of heterosexually acquired HIV infection: a meta-analysis of randomized trials involving 11,050 men. HIV Med. 2008;9:332–335. - PubMed
-
- Steinbrook R. One step forward, two steps back–will there ever be an AIDS vaccine? N Engl J Med. 2007;357:2653–2655. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

