Skeletal muscle phenotypically converts and selectively inhibits metastatic cells in mice
- PMID: 20174581
- PMCID: PMC2823787
- DOI: 10.1371/journal.pone.0009299
Skeletal muscle phenotypically converts and selectively inhibits metastatic cells in mice
Abstract
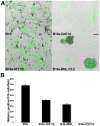
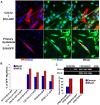
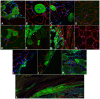
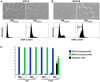
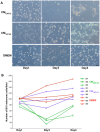
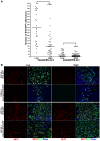
Skeletal muscle is rarely a site of malignant metastasis; the molecular and cellular basis for this rarity is not understood. We report that myogenic cells exert pronounced effects upon co-culture with metastatic melanoma (B16-F10) or carcinoma (LLC1) cells including conversion to the myogenic lineage in vitro and in vivo, as well as inhibition of melanin production in melanoma cells coupled with cytotoxic and cytostatic effects. No effect is seen with non-tumorigenic cells. Tumor suppression assays reveal that the muscle-mediated tumor suppressor effects do not generate resistant clones but function through the down-regulation of the transcription factor MiTF, a master regulator of melanocyte development and a melanoma oncogene. Our findings point to skeletal muscle as a source of therapeutic agents in the treatment of metastatic cancers.
Conflict of interest statement
Figures
Similar articles
-
Clone-derived human AF-amniotic fluid stem cells are capable of skeletal myogenic differentiation in vitro and in vivo.J Tissue Eng Regen Med. 2012 Aug;6(8):598-613. doi: 10.1002/term.462. Epub 2012 Mar 7. J Tissue Eng Regen Med. 2012. PMID: 22396316
-
Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering.BMC Cell Biol. 2017 Feb 28;18(1):15. doi: 10.1186/s12860-017-0131-2. BMC Cell Biol. 2017. PMID: 28245809 Free PMC article.
-
Conversion of dermal fibroblasts to a myogenic lineage is induced by a soluble factor derived from myoblasts.J Cell Biochem. 1996 Jun 1;61(3):363-74. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C363::AID-JCB4%3E3.0.CO;2-R. J Cell Biochem. 1996. PMID: 8761941
-
Dedifferentiation of adult human myoblasts induced by ciliary neurotrophic factor in vitro.Mol Biol Cell. 2005 Jul;16(7):3140-51. doi: 10.1091/mbc.e05-03-0218. Epub 2005 Apr 20. Mol Biol Cell. 2005. PMID: 15843428 Free PMC article.
-
Effects of Creatine in Skeletal Muscle Cells and in Myoblasts Differentiating Under Normal or Oxidatively Stressing Conditions.Mini Rev Med Chem. 2016;16(1):4-11. doi: 10.2174/1389557515666150722102342. Mini Rev Med Chem. 2016. PMID: 26202198 Review.
Cited by
-
Role of pericytes in skeletal muscle regeneration and fat accumulation.Stem Cells Dev. 2013 Aug 15;22(16):2298-314. doi: 10.1089/scd.2012.0647. Epub 2013 Apr 27. Stem Cells Dev. 2013. PMID: 23517218 Free PMC article.
-
Muscle Stem Cells Give Rise to Rhabdomyosarcomas in a Severe Mouse Model of Duchenne Muscular Dystrophy.Cell Rep. 2019 Jan 15;26(3):689-701.e6. doi: 10.1016/j.celrep.2018.12.089. Cell Rep. 2019. PMID: 30650360 Free PMC article.
-
Decoding melanoma metastasis.Cancers (Basel). 2010 Dec 30;3(1):126-63. doi: 10.3390/cancers3010126. Cancers (Basel). 2010. PMID: 24212610 Free PMC article.
-
Scalp melanoma with rectus abdominis metastasis: A rare case report.Medicine (Baltimore). 2019 Jul;98(28):e16395. doi: 10.1097/MD.0000000000016395. Medicine (Baltimore). 2019. PMID: 31305447 Free PMC article.
-
Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation.Proc Natl Acad Sci U S A. 2015 Jan 6;112(1):214-9. doi: 10.1073/pnas.1417115112. Epub 2014 Dec 18. Proc Natl Acad Sci U S A. 2015. PMID: 25524628 Free PMC article.
References
-
- Fidler IJ. Critical determinants of cancer metastasis: rationale for therapy. Cancer Chemother Pharmacol. 1999;43(Suppl):S3–10. - PubMed
-
- Greig RG, Trainer DL. Shaping future strategies for the pharmacological control of tumor cell metastases. Cancer Metastasis Rev. 1986;5:3–14. - PubMed
-
- Klein CA. The direct molecular analysis of metastatic precursor cells in breast cancer: A chance for a better understanding of metastasis and for personalised medicine. Eur J Cancer 2008 - PubMed
-
- Langley RR, Fidler IJ. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 2007;28:297–321. - PubMed
-
- Crissman JD. Tumor-host interactions as prognostic factors in the histologic assessment of carcinomas. Pathol Annu. 1986;21 Pt 1:29–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

