A new nuclear function of the Entamoeba histolytica glycolytic enzyme enolase: the metabolic regulation of cytosine-5 methyltransferase 2 (Dnmt2) activity
- PMID: 20174608
- PMCID: PMC2824750
- DOI: 10.1371/journal.ppat.1000775
A new nuclear function of the Entamoeba histolytica glycolytic enzyme enolase: the metabolic regulation of cytosine-5 methyltransferase 2 (Dnmt2) activity
Abstract
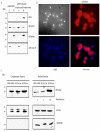
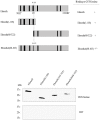
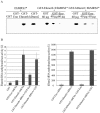
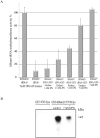
Cytosine-5 methyltransferases of the Dnmt2 family function as DNA and tRNA methyltransferases. Insight into the role and biological significance of Dnmt2 is greatly hampered by a lack of knowledge about its protein interactions. In this report, we address the subject of protein interaction by identifying enolase through a yeast two-hybrid screen as a Dnmt2-binding protein. Enolase, which is known to catalyze the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP), was shown to have both a cytoplasmatic and a nuclear localization in the parasite Entamoeba histolytica. We discovered that enolase acts as a Dnmt2 inhibitor. This unexpected inhibitory activity was antagonized by 2-PG, which suggests that glucose metabolism controls the non-glycolytic function of enolase. Interestingly, glucose starvation drives enolase to accumulate within the nucleus, which in turn leads to the formation of additional enolase-E.histolytica DNMT2 homolog (Ehmeth) complex, and to a significant reduction of the tRNA(Asp) methylation in the parasite. The crucial role of enolase as a Dnmt2 inhibitor was also demonstrated in E.histolytica expressing a nuclear localization signal (NLS)-fused-enolase. These results establish enolase as the first Dnmt2 interacting protein, and highlight an unexpected role of a glycolytic enzyme in the modulation of Dnmt2 activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Spada F, Rothbauer U, Zolghadr K, Schermelleh L, Leonhardt H. Regulation of DNA methyltransferase 1. Adv Enzyme Regul. 2006;46:224–234. - PubMed
-
- Jeltsch A. Molecular enzymology of mammalian DNA methyltransferases. Curr Top Microbiol Immunol. 2006;301:203–225. - PubMed
-
- Goll MG, Kirpekar F, Maggert KA, Yoder JA, Hsieh CL, et al. Methylation of tRNAAsp by the DNA methyltransferase homolog Dnmt2. Science. 2006;311:395–398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

