Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine
- PMID: 20174647
- PMCID: PMC2822849
- DOI: 10.1371/journal.pone.0009260
Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine
Abstract
Background: Non-motor symptoms (e.g., depression, anxiety, and cognitive deficits) in patients with Parkinson disease (PD) precede the onset of the motor symptoms. Although these symptoms do not respond to pharmacological dopamine replacement therapy, their precise pathological mechanisms are currently unclear. The present study was undertaken to examine whether the unilateral 6-hydroxydopamine (6-OHDA) lesion to the substantia nigra pars compacta (SNc), which represents a model of long-term dopaminergic neurotoxicity, could affect cell proliferation in the adult rat brain. Furthermore, we examined the effects of the selective serotonin reuptake inhibitor (SSRI) fluoxetine and the selective noradrenaline reuptake inhibitor maprotiline on the reduction in cell proliferation in the subgranular zone (SGZ) by the unilateral 6-OHDA lesion.
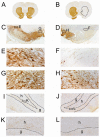
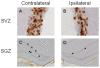
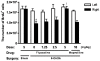
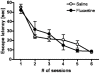
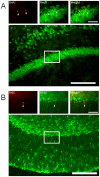
Methodology/principal findings: A single unilateral injection of 6-OHDA into the rat SNc resulted in an almost complete loss of tyrosine hydroxylase (TH) immunoreactivity in the striatum and SNc, as well as in reductions of TH-positive cells and fibers in the ventral tegmental area (VTA). On the other hand, an injection of vehicle alone showed no overt change in TH immunoreactivity. A unilateral 6-OHDA lesion to SNc significantly decreased cell proliferation in the SGZ ipsilateral to the 6-OHDA lesion, but not in the contralateral SGZ or the subventricular zone (SVZ), of rats. Furthermore, subchronic (14 days) administration of fluoxetine (5 mg/kg/day), but not maprotiline significantly attenuated the reduction in cell proliferation in the SGZ by unilateral 6-OHDA lesion.
Conclusions/significance: The present study suggests that cell proliferation in the SGZ of the dentate gyrus might be, in part, under dopaminergic control by SNc and VTA, and that subchronic administration of fluoxetine reversed the reduction in cell proliferation in the SGZ by 6-OHDA. Therefore, SSRIs such as fluoxetine might be potential therapeutic drugs for non-motor symptoms as well as motor symptoms in patients with PD, which might be associated with the reduction in cell proliferation in the SGZ.
Conflict of interest statement
Figures
Similar articles
-
Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson's disease.Neuroscience. 2008 Feb 19;151(4):1142-53. doi: 10.1016/j.neuroscience.2007.11.036. Epub 2007 Dec 4. Neuroscience. 2008. PMID: 18201835
-
Dopamine cell morphology and glial cell hypertrophy and process branching in the nigrostriatal system after striatal 6-OHDA analyzed by specific sterological tools.Int J Neurosci. 2005 Apr;115(4):557-82. doi: 10.1080/00207450590521118. Int J Neurosci. 2005. PMID: 15804725
-
Astroglial and microglial activation in the wistar rat ventral tegmental area after a single striatal injection of 6-hydroxydopamine.Int J Neurosci. 2004 Feb;114(2):197-216. doi: 10.1080/00207450490249338. Int J Neurosci. 2004. PMID: 14702208
-
Involvement of astroglial fibroblast growth factor-2 and microglia in the nigral 6-OHDA parkinsonism and a possible role of glucocorticoid hormone on the glial mediated local trophism and wound repair.J Neural Transm Suppl. 2009;(73):185-202. doi: 10.1007/978-3-211-92660-4_15. J Neural Transm Suppl. 2009. PMID: 20411778
-
Ion Channel and Neurotransmitter Modulators as Electroceutical Approaches to the Control of Cancer.Curr Pharm Des. 2017;23(32):4827-4841. doi: 10.2174/1381612823666170530105837. Curr Pharm Des. 2017. PMID: 28554310 Free PMC article. Review.
Cited by
-
Synucleinopathies: common features and hippocampal manifestations.Cell Mol Life Sci. 2017 Apr;74(8):1485-1501. doi: 10.1007/s00018-016-2411-y. Epub 2016 Nov 8. Cell Mol Life Sci. 2017. PMID: 27826641 Free PMC article. Review.
-
Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model.Eur J Neurosci. 2012 Jan;35(1):10-9. doi: 10.1111/j.1460-9568.2011.07933.x. Eur J Neurosci. 2012. PMID: 22211740 Free PMC article.
-
Commentary: Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors.Front Mol Neurosci. 2023 Feb 8;16:1117121. doi: 10.3389/fnmol.2023.1117121. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36846567 Free PMC article. No abstract available.
-
Intranigral Injection of Endotoxin Suppresses Proliferation of Hippocampal Progenitor Cells.Front Neurosci. 2019 Jul 3;13:687. doi: 10.3389/fnins.2019.00687. eCollection 2019. Front Neurosci. 2019. PMID: 31333405 Free PMC article.
-
Adult human neurogenesis: from microscopy to magnetic resonance imaging.Front Neurosci. 2011 Apr 4;5:47. doi: 10.3389/fnins.2011.00047. eCollection 2011. Front Neurosci. 2011. PMID: 21519376 Free PMC article.
References
-
- Samii A, Nutt JG, Ransom BR. Parkinson's disease. Lancet. 2004;363:1783–1793. - PubMed
-
- Schrag A, Achott M. Epidemiological, clinical, and genetic characteristics of early-onset parkinsonism. Lancet Neurol. 2006;5:355–363. - PubMed
-
- Weintraub D, Comella CL, Horn S. Parkinson's disease - Part 1: Pathophysiology, symptoms, burden, diagnosis, and assessment. Am J Mnag Care. 2008;14:S40–S48. - PubMed
-
- Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, et al. Subclinical dopaminergic dysfunction in asymptomatic Parkinson's disease patients' relatives with a decreased sense of smell. Ann Neurol. 2001;50:34–41. - PubMed
-
- Oertel WH, Höglinger GU, Caraceni T, Girotti F, Eichhorn T, et al. Depression in Parkinson's disease. An update. Adv Neurol. 2001;86:373–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

