Developmental sex differences in nicotinic currents of prefrontal layer VI neurons in mice and rats
- PMID: 20174655
- PMCID: PMC2822857
- DOI: 10.1371/journal.pone.0009261
Developmental sex differences in nicotinic currents of prefrontal layer VI neurons in mice and rats
Abstract
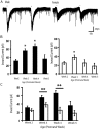
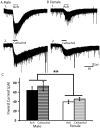
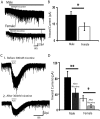
Background: There is a large sex difference in the prevalence of attention deficit disorder; yet, relatively little is known about sex differences in the development of prefrontal attention circuitry. In male rats, nicotinic acetylcholine receptors excite corticothalamic neurons in layer VI, which are thought to play an important role in attention by gating the sensitivity of thalamic neurons to incoming stimuli. These nicotinic currents in male rats are significantly larger during the first postnatal month when prefrontal circuitry is maturing. The present study was undertaken to investigate whether there are sex differences in the nicotinic currents in prefrontal layer VI neurons during development.
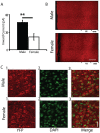
Methodology/principal findings: Using whole cell recording in prefrontal brain slice, we examined the inward currents elicited by nicotinic stimulation in male and female rats and two strains of mice. We found a prominent sex difference in the currents during the first postnatal month when males had significantly greater nicotinic currents in layer VI neurons compared to females. These differences were apparent with three agonists: acetylcholine, carbachol, and nicotine. Furthermore, the developmental sex difference in nicotinic currents occurred despite male and female rodents displaying a similar pattern and proportion of layer VI neurons possessing a key nicotinic receptor subunit.
Conclusions/significance: This is the first illustration at a cellular level that prefrontal attention circuitry is differently affected by nicotinic receptor stimulation in males and females during development. This transient sex difference may help to define the cellular and circuit mechanisms that underlie vulnerability to attention deficit disorder.
Conflict of interest statement
Figures
References
-
- Brown RT, Freeman WS, Perrin JM, Stein MT, Amler RW, et al. Prevalence and assessment of attention-deficit/hyperactivity disorder in primary care settings. Pediatrics. 2001;107:E43. - PubMed
-
- Cuffe SP, Moore CG, McKeown RE. Prevalence and correlates of ADHD symptoms in the national health interview survey. Journal of attention disorders. 2005;9:392–401. - PubMed
-
- Smalley SL, McGough JJ, Moilanen IK, Loo SK, Taanila A, et al. Prevalence and psychiatric comorbidity of attention-deficit/hyperactivity disorder in an adolescent Finnish population. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:1575–1583. - PubMed
-
- Sullivan RM, Brake WG. What the rodent prefrontal cortex can teach us about attention-deficit/hyperactivity disorder: the critical role of early developmental events on prefrontal function. Behav Brain Res. 2003;146:43–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

