Modified RECIST (mRECIST) assessment for hepatocellular carcinoma
- PMID: 20175033
- PMCID: PMC12268942
- DOI: 10.1055/s-0030-1247132
Modified RECIST (mRECIST) assessment for hepatocellular carcinoma
Abstract
The endpoint in cancer research is overall survival. Nonetheless, other potential surrogate endpoints, such as response rate and time to progression, are currently used. Measurement of response rate in hepatocellular carcinoma (HCC) has become a controversial issue. The World Health Organization (WHO) criteria underestimate the actual response rate; thus, they were amended in 2000 by a panel of experts convened by the European Association for the Study of the Liver (EASL) to take into account treatment-induced tumor necrosis. Applying these guidelines, there was an association between response rate and outcome prediction. More recently, the Response Evaluation Criteria in Solid Tumors (RECIST) guideline was proposed as a method for measuring treatment response based on tumor shrinkage, which is a valuable measure of antitumor activity of cytotoxic drugs. This method was initially adopted by regulatory agencies, such as the U.S. Food and Drug Administration (FDA), for drug approval. However, anatomic tumor response metrics can be misleading when applied to molecular-targeted therapies or locoregional therapies in HCC. In 2008, a group of experts convened by the American Association for the Study of Liver Diseases (AASLD) developed a set of guidelines aimed at providing a common framework for the design of clinical trials in HCC and adapted the concept of viable tumor-tumoral tissue showing uptake in arterial phase of contrast-enhanced radiologic imaging techniques-to formally amend RECIST. These amendments conformed the AASLD-JNCI (Journal of the National Cancer Institute) guidelines and are summarized and clarified in the current article. They are referred to herein as the modified RECIST assessment (mRECIST). Further studies are needed to confirm the accuracy of this measurement compared with conventional gold standards such as pathologic studies of explanted livers.
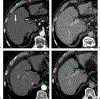
Figures

References
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47(1): 207–214 - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92(3):205–216 - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. ; SHARP Investigators Study Group. Sorafenib in advanced hepato-cellular carcinoma. N Engl J Med 2008;359(4):378–390 - PubMed
-
- Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 2009;115(3):616–623 - PubMed
-
- Bruix J, Sherman M, Llovet JM, et al. ; EASL Panel of Experts on HCCEuropean Association for the Study of the Liver. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. J Hepatol 2001;35(3):421–430 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

