Cancer models in Caenorhabditis elegans
- PMID: 20175192
- PMCID: PMC4098942
- DOI: 10.1002/dvdy.22247
Cancer models in Caenorhabditis elegans
Abstract
Although now dogma, the idea that nonvertebrate organisms such as yeast, worms, and flies could inform, and in some cases even revolutionize, our understanding of oncogenesis in humans was not immediately obvious. Aided by the conservative nature of evolution and the persistence of a cohort of devoted researchers, the role of model organisms as a key tool in solving the cancer problem has, however, become widely accepted. In this review, we focus on the nematode Caenorhabditis elegans and its diverse and sometimes surprising contributions to our understanding of the tumorigenic process. Specifically, we discuss findings in the worm that address a well-defined set of processes known to be deregulated in cancer cells including cell cycle progression, growth factor signaling, terminal differentiation, apoptosis, the maintenance of genome stability, and developmental mechanisms relevant to invasion and metastasis.
Copyright (c) 2010 Wiley-Liss, Inc.
Figures
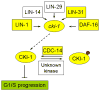
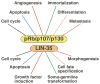


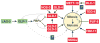



References
-
- Abdel-Rahman WM, Peltomaki P. Lynch syndrome and related familial colorectal cancers. Crit Rev Oncog. 2008;14:1–22. discussion 23–31. - PubMed
-
- Abedi H, Zachary I. Signalling mechanisms in the regulation of vascular cell migration. Cardiovasc Res. 1995;30:544–556. - PubMed
-
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell. 2003;4:11–18. - PubMed
-
- Ahmed S, Hodgkin J. MRT-2 checkpoint protein is required for germline immortality and telomere replication in C. elegans. Nature. 2000;403:159–164. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

