A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia
- PMID: 20175406
- PMCID: PMC2817909
- DOI: 10.1093/sleep/33.2.225
A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia
Abstract
Background: Longer-term pharmacologic studies for insomnia in older individuals are sparse.
Objective: To evaluate the efficacy and safety of 12 weeks of nightly eszopiclone in elderly outpatients with insomnia.
Methods: Participants (65-85 years) met DSM-IV-TR criteria for insomnia with total sleep times (TST) < or = 6 h, and wake time after sleep onset (WASO) > or = 45 min. Participants were randomized to 12 weeks of eszopiclone 2 mg (n = 194) or placebo (n = 194), followed by a 2-week single-blind placebo run-out. Subject-reported measures of sleep (sTST, sleep latency [sSL], sWASO) and daytime function (alertness, concentration, wellbeing, ability to function) were assessed. AEs were monitored.
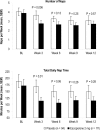
Results: Subjects treated with 2 mg eszopiclone slept longer at night on average and at every individual time point compared to baseline than placebo subjects, as measured by TST over the 12-week double-blind period (P < 0.0001). Mean sTST over the double-blind period for eszopiclone-treated subjects was 360.08 min compared to 297.86 min at baseline, a mean change of 63.24 min. Over the double-blind period, eszopiclone-treated subjects also experienced a significantly greater improvement in sSL compared to placebo, with a mean decrease of 24.62 min versus a mean decrease of 19.92 min, respectively (P = 0.0014). Eszopiclone subjects also experienced a significantly greater decrease in WASO (mean decrease of 36.4 min) compared to placebo subjects (decrease of 14.8 min) (P < 0.0001). Post-discontinuation, sleep parameters were statistically improved versus baseline for eszopiclone (P-values < or = 0.01), indicating no rebound. The most common AEs (> or = 5%) were headache (eszopiclone 13.9%, placebo 12.4%), unpleasant taste (12.4%, 1.5%), and nasopharyngitis (5.7%, 6.2%).
Conclusion: In this Phase IV trial of older adults with insomnia, eszopiclone significantly improved patient-reported sleep and daytime function relative to placebo. Improvements occurred within the first week and were maintained for 3 months, with no evidence of rebound insomnia following discontinuation. The 12 weeks of treatment were well tolerated.
Clinical trial information: A Long-Term Safety and Efficacy Study of Eszopiclone in Elderly Subjects With Primary Chronic Insomnia; Registration #NCT00386334; URL - http://www.clinicaltrials.gov/ct2/show/NCT00386334?term=eszopiclone&rank=24
Figures



Similar articles
-
Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia.Sleep. 2003 Nov 1;26(7):793-9. doi: 10.1093/sleep/26.7.793. Sleep. 2003. PMID: 14655910 Clinical Trial.
-
A polysomnography study of eszopiclone in elderly patients with insomnia.Curr Med Res Opin. 2006 Sep;22(9):1633-42. doi: 10.1185/030079906X112741. Curr Med Res Opin. 2006. PMID: 16968566 Clinical Trial.
-
An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia.Sleep Med. 2005 Nov;6(6):487-95. doi: 10.1016/j.sleep.2005.06.004. Epub 2005 Oct 17. Sleep Med. 2005. PMID: 16230048 Clinical Trial.
-
Eszopiclone for the treatment of primary insomnia: a systematic review and meta-analysis of double-blind, randomized, placebo-controlled trials.Sleep Med. 2019 Oct;62:6-13. doi: 10.1016/j.sleep.2019.03.016. Epub 2019 Apr 6. Sleep Med. 2019. PMID: 31518944
-
Eszopiclone: a review of its use in the treatment of insomnia.Drugs. 2008;68(10):1415-34. doi: 10.2165/00003495-200868100-00005. Drugs. 2008. PMID: 18578559 Review.
Cited by
-
A randomized, double-blind, placebo-controlled trial of eszopiclone for the treatment of insomnia in patients with chronic low back pain.Sleep. 2014 Jun 1;37(6):1053-60. doi: 10.5665/sleep.3760. Sleep. 2014. PMID: 24882900 Free PMC article. Clinical Trial.
-
Association between psychotropics use and occurrence of falls in hospitalized patients: A matched case-control study.PCN Rep. 2023 Aug 13;2(3):e133. doi: 10.1002/pcn5.133. eCollection 2023 Sep. PCN Rep. 2023. PMID: 38867824 Free PMC article.
-
Identification of the simultaneous use of multiple hypnotics as a risk factor for falls in hospitalized patients by a matched case-control study.PLoS One. 2023 Sep 19;18(9):e0291607. doi: 10.1371/journal.pone.0291607. eCollection 2023. PLoS One. 2023. PMID: 37725607 Free PMC article.
-
A randomized controlled trial of mindfulness meditation for chronic insomnia.Sleep. 2014 Sep 1;37(9):1553-63. doi: 10.5665/sleep.4010. Sleep. 2014. PMID: 25142566 Free PMC article. Clinical Trial.
-
Insomnia in Elderly Patients: Recommendations for Pharmacological Management.Drugs Aging. 2018 Sep;35(9):791-817. doi: 10.1007/s40266-018-0569-8. Drugs Aging. 2018. PMID: 30058034 Review.
References
-
- Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. I. Sleep. 1999;22:S347–353. - PubMed
-
- Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J Clin Psychiatry. 2005;66(Suppl 9):10–13. - PubMed
-
- Mellinger GD, Balter MD, Uhlenhuth EH. Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry. 1985;2:225–32. - PubMed
-
- Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. - PubMed
-
- Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Sleep Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22:S366–372. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

