A novel insulin secretagogue based on a dinucleoside polyphosphate scaffold
- PMID: 20175517
- PMCID: PMC4363086
- DOI: 10.1021/jm901621h
A novel insulin secretagogue based on a dinucleoside polyphosphate scaffold
Abstract
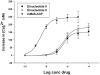
Dinucleoside polyphosphates exert their physiological effects via P2 receptors (P2Rs). They are attractive drug candidates, as they offer better stability and specificity compared to nucleotides, the most common P2 receptor ligands. The activation of pancreatic P2Y receptors by nucleotides increases insulin secretion. Therefore, in the current study, dinucleoside polyphosphate analogues (di-(2-MeS)-adenosine-5',5''-P(1),P(4),alpha,beta-methylene-tetraphosphate), 8, (di-(2-MeS)-adenosine-5',5''-P(1),P(4),beta,gamma-methylene-tetraphosphate), 9, and di-(2-MeS)-adenosine-5',5''-P(1),P(3),alpha,beta-methylene triphosphate, 10, were developed as potential insulin secretagogues. Analogues 8 and 9 were found to be agonists of the P2Y(1)R with EC(50) values of 0.42 and 0.46 microM, respectively, whereas analogue 10 had no activity. Analogues 8-10 were found to be completely resistant to hydrolysis by alkaline phosphatase over 3 h at 37 degrees C. Analogue 8 also was found to be 2.5-fold more stable in human blood serum than ATP, with a half-life of 12.1 h. Analogue 8 administration in rats caused a decrease in a blood glucose load from 155 mg/dL to ca. 100 mg/dL and increased blood insulin levels 4-fold as compared to basal levels. In addition, analogue 8 reduced a blood glucose load to normal values (80-110 mg/dL), unlike the commonly prescribed glibenclamide, which reduced glucose levels below normal values (60 mg/dL). These findings suggest that analogue 8 may prove to be an effective and safe treatment for type 2 diabetes.
Figures





Similar articles
-
Diadenosine and diuridine poly(borano)phosphate analogues: synthesis, chemical and enzymatic stability, and activity at P2Y1 and P2Y2 receptors.J Med Chem. 2006 Mar 23;49(6):1980-90. doi: 10.1021/jm050955y. J Med Chem. 2006. PMID: 16539385
-
Boranophosphate isoster controls P2Y-receptor subtype selectivity and metabolic stability of dinucleoside polyphosphate analogues.J Med Chem. 2012 Jan 12;55(1):437-48. doi: 10.1021/jm2013198. Epub 2011 Dec 7. J Med Chem. 2012. PMID: 22107038 Free PMC article.
-
Pyrimidine ribonucleotides with enhanced selectivity as P2Y(6) receptor agonists: novel 4-alkyloxyimino, (S)-methanocarba, and 5'-triphosphate gamma-ester modifications.J Med Chem. 2010 Jun 10;53(11):4488-501. doi: 10.1021/jm100287t. J Med Chem. 2010. PMID: 20446735 Free PMC article.
-
Purinergic receptors on insulin-secreting cells.Fundam Clin Pharmacol. 1994;8(2):117-27. doi: 10.1111/j.1472-8206.1994.tb00788.x. Fundam Clin Pharmacol. 1994. PMID: 8020870 Review.
-
P2 purinergic signalling in the pancreatic beta-cell: control of insulin secretion and pharmacology.Eur J Pharm Sci. 2009 May 12;37(2):67-75. doi: 10.1016/j.ejps.2009.01.007. Epub 2009 Jan 30. Eur J Pharm Sci. 2009. PMID: 19429412 Review.
Cited by
-
Identification of a promising drug candidate for the treatment of type 2 diabetes based on a P2Y(1) receptor agonist.J Med Chem. 2012 Sep 13;55(17):7623-35. doi: 10.1021/jm3006355. Epub 2012 Aug 20. J Med Chem. 2012. PMID: 22873688 Free PMC article.
-
Molecular Structure of P2Y Receptors: Mutagenesis, Modeling, and Chemical Probes.Wiley Interdiscip Rev Membr Transp Signal. 2012 Sep 12;1(6):WMTS68. doi: 10.1002/wmts.68. Wiley Interdiscip Rev Membr Transp Signal. 2012. PMID: 23336097 Free PMC article.
-
G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions.Purinergic Signal. 2012 Sep;8(3):419-36. doi: 10.1007/s11302-012-9294-7. Epub 2012 Feb 29. Purinergic Signal. 2012. PMID: 22371149 Free PMC article. Review.
-
Purinergic signalling and diabetes.Purinergic Signal. 2013 Sep;9(3):307-24. doi: 10.1007/s11302-013-9359-2. Epub 2013 Apr 3. Purinergic Signal. 2013. PMID: 23546842 Free PMC article. Review.
-
Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells.Biochem Pharmacol. 2011 Aug 15;82(4):418-25. doi: 10.1016/j.bcp.2011.05.013. Epub 2011 May 23. Biochem Pharmacol. 2011. PMID: 21632028 Free PMC article.
References
-
- Fischer B, Nahum V. U.S. Patent 7,368,439. Dinucleoside poly(borano)phosphate derivatives and uses thereof. 2008
-
- Ashcroft F, Rorsman P. Type 2 diabetes mellitus: not quite exciting enough? Hum Mol Genet. 2004;13:R21–R31. - PubMed
-
- Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. - PubMed
-
- UPDSU Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352:837–853. - PubMed
-
- Ishida H. Mechanism of action of antidiabetic sulfonylureas. Diabetes Front. 1999;10:99–104.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical
Miscellaneous

