Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies
- PMID: 20175775
- PMCID: PMC2914575
- DOI: 10.1111/j.1365-2141.2010.08097.x
Exercise capacity and haemodynamics in patients with sickle cell disease with pulmonary hypertension treated with bosentan: results of the ASSET studies
Abstract
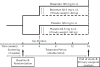
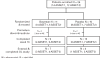
Doppler-defined pulmonary hypertension (PH) in sickle cell disease (SCD) is associated with 40% mortality at 40 months. To assess the effect of bosentan in SCD-PH, two randomized, double-blind, placebo-controlled, 16-week studies were initiated. Safety concerns are particularly relevant in SCD due to comorbid conditions. ASSET-1 and -2 enrolled patients with pulmonary arterial hypertension (PAH) and pulmonary venous hypertension (PH), respectively. Haemodynamics and 6-min walk distance (6MWD) were obtained at baseline and week 16. The studies were terminated due to slow site initiation and patient enrolment (n = 26). Bosentan appeared to be well tolerated. Although sample sizes were limited, in ASSET-1 at baseline, 6MWD correlated with cardiac output (CO; P = 0.006) with non-significant inverse correlations between 6MWD and pulmonary vascular resistance (PVR; P = 0.07) and between 6MWD and right atrial pressure (P = 0.08). In ASSET-2 at baseline, there was a non-significant correlation between 6MWD and CO (P = 0.06). Due to limited sample sizes, efficacy endpoints were not analysed. However, in both studies, non-significant increases in CO were observed with bosentan compared to placebo. Similarly, non-significant decreases in PVR were observed with bosentan. Limited data in SCD-PH suggest that a low 6MWD predicts a low CO. Standard-dose bosentan appears to be well tolerated. Further investigation is warranted. Clinicaltrials.gov registration numbers NCT00310830, NCT00313196, NCT00360087.
Conflict of interest statement
Figures













References
-
- Anthi A, Machado RF, Jison ML, Taveira-Dasilva AM, Rubin LJ, Hunter L, Hunter CJ, Coles W, Nichols J, Avila NA, Sachdev V, Chen CC, Gladwin MT. Hemodynamic and functional assessment of patients with sickle cell disease and pulmonary hypertension. Am J Respir Crit Care Med. 2007;175:1272–1279. - PMC - PubMed
-
- Ataga KI, Sood N, De Gent G, Kelly E, Henderson AG, Jones S, Strayhorn D, Lail A, Lieff S, Orringer EP. Pulmonary hypertension in sickle cell disease. Am J Med. 2004;117:665–669. - PubMed
-
- Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–115. - PubMed
-
- Barst RJ, Rich S, Widlitz A, Horn EM, McLaughlin V, McFarlin J. Clinical efficacy of sitaxsentan, an endothelin-A receptor antagonist, in patients with pulmonary arterial hypertension: open-label pilot study. Chest. 2002;121:1860–1868. - PubMed
-
- Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

