Analysis of inhibition of topoisomerase IIalpha and cancer cell proliferation by ingenolEZ
- PMID: 20175785
- PMCID: PMC11158357
- DOI: 10.1111/j.1349-7006.2009.01408.x
Analysis of inhibition of topoisomerase IIalpha and cancer cell proliferation by ingenolEZ
Abstract
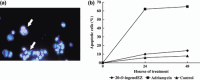
We previously reported that many ingenol compounds derived from Euphorbia kansui exhibit topoisomerase inhibitory activity and/or inhibitory activity of cell proliferation. The inhibitory effects of 20-O-(2'E,4'Z-decadienoyl) ingenol and 3-O-(2'E,4'Z-decadienoyl)-ingenol among these compounds on topoisomerase II activity and on the cell proliferative activity and arrest phase of the cell cycle were studied using a mouse breast cancer (MMT) cell line. Although 20-O-ingenolEZ exerted inhibitory effects on both topoisomerase II activity and cell proliferative activity, 3-O-ingenolEZ exerted inhibitory activity on neither. The 20-O-ingenolEZ-induced cell arrest of MMT-cell proliferation led to a cell cycle arrest in the G2/M phase. Topoisomerase II inhibition can be divided into the poison and catalytic inhibitor types. A checkpoint mechanism is activated when cells are treated with these topoisomerase II inhibitors. Poison-type inhibition occurs via induction of the DNA damage checkpoint and the catalytic-type inhibition occurs via induction of the DNA-decatenation checkpoint, suggestive of distinct checkpoint reactions. 20-O-ingenolEZ inhibited topoisomerase IIalpha activity through inhibition of ATPase, and induced DNA-decatenation checkpoint without signaling for phosphorylation of H2AX.
Figures






Similar articles
-
Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus.J Nat Prod. 2002 Sep;65(9):1246-51. doi: 10.1021/np0200921. J Nat Prod. 2002. PMID: 12350140
-
The water expelling effect evaluation of 3-O-(2'E,4'Z-decadienoyl)-20-O-acetylingenol and ingenol on H22 mouse hepatoma ascites model and their content differences analysis in Euphorbia kansui before and after stir-fried with vinegar by UPLC.J Ethnopharmacol. 2021 Mar 1;267:113507. doi: 10.1016/j.jep.2020.113507. Epub 2020 Oct 22. J Ethnopharmacol. 2021. PMID: 33098970
-
3-O-(2'E,4'Z-decadienoyl)-20-O-acetylingenol induces apoptosis in intestinal epithelial cells of rats via mitochondrial pathway.J Ethnopharmacol. 2015 Nov 4;174:331-8. doi: 10.1016/j.jep.2015.08.036. Epub 2015 Aug 28. J Ethnopharmacol. 2015. PMID: 26318745
-
Ultra-performance liquid chromatography-quadrupole/time-of-flight mass spectrometry analysis of the impact of processing on toxic components of Kansui Radix.BMC Complement Altern Med. 2016 Feb 24;16:73. doi: 10.1186/s12906-016-1039-7. BMC Complement Altern Med. 2016. PMID: 26912002 Free PMC article.
-
Inhibition of topoisomerase IIalpha and G2 cell cycle arrest by NK314, a novel benzo[c]phenanthridine currently in clinical trials.Mol Cancer Ther. 2007 May;6(5):1501-8. doi: 10.1158/1535-7163.MCT-06-0780. Mol Cancer Ther. 2007. PMID: 17513599
Cited by
-
3EZ,20Ac-ingenol, a catalytic inhibitor of topoisomerases, downregulates p-Akt and induces DSBs and apoptosis of DT40 cells.Arch Pharm Res. 2013 Aug;36(8):1029-38. doi: 10.1007/s12272-013-0108-4. Epub 2013 Apr 18. Arch Pharm Res. 2013. PMID: 23595550 Free PMC article.
-
Recent Progress of Research on Herbal Products Used in Traditional Chinese Medicine: the Herbs belonging to The Divine Husbandman's Herbal Foundation Canon ( Shén Nóng Běn Cǎo Jīng).J Tradit Complement Med. 2012 Jan;2(1):6-26. doi: 10.1016/s2225-4110(16)30066-9. J Tradit Complement Med. 2012. PMID: 24716110 Free PMC article. Review.
-
Copper Complexes as Anticancer Agents Targeting Topoisomerases I and II.Cancers (Basel). 2020 Oct 5;12(10):2863. doi: 10.3390/cancers12102863. Cancers (Basel). 2020. PMID: 33027952 Free PMC article. Review.
References
-
- Miyata S, Wang LY, Yoshida C et al. Inhibition of cellular proliferation by diterpenes, topoisomerase II inhibitor. Bioorg Med Chem 2006; 14: 2048–51. - PubMed
-
- Wu TS, Lin YM, Haruna M et al. Antitumor agents, 119. Kansuiphorins A and B, two novel antileukemic diterpene esters from Euphorbia kansui . J Nat Prod 1991; 54: 823–9. - PubMed
-
- Yu FR, Hou XL, Chen J. Study of inhibition and antioxidation of Mao Eryan extract on L615 leukemia cell. J Lanzhou Univ 2003; 39: 453–60.
-
- Zheng WF, Cui Z, Zhu Q. Cytotoxicity and antiviral activity of the compounds from Euphorbia kansui . Planta Med 1998; 64: 754–6. - PubMed
-
- Whelan LC, Ryan MF. Ethanolic extracts of Euphorbia and other ethnobotanical species as inhibitors of human tumour cell growth. Phytomedicine 2003; 10: 53–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

