Dynamics of muscle fibre growth during postnatal mouse development
- PMID: 20175910
- PMCID: PMC2836990
- DOI: 10.1186/1471-213X-10-21
Dynamics of muscle fibre growth during postnatal mouse development
Abstract
Background: Postnatal growth in mouse is rapid, with total skeletal muscle mass increasing several-fold in the first few weeks. Muscle growth can be achieved by either an increase in muscle fibre number or an increase in the size of individual myofibres, or a combination of both. Where myofibre hypertrophy during growth requires the addition of new myonuclei, these are supplied by muscle satellite cells, the resident stem cells of skeletal muscle.
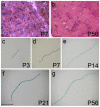
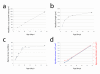
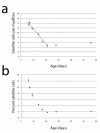
Results: Here, we report on the dynamics of postnatal myofibre growth in the mouse extensor digitorum longus (EDL) muscle, which is essentially composed of fast type II fibres in adult. We found that there was no net gain in myofibre number in the EDL between P7 and P56 (adulthood). However, myofibre cross-sectional area increased by 7.6-fold, and length by 1.9-fold between these ages, resulting in an increase in total myofibre volume of 14.1-fold: showing the extent of myofibre hypertrophy during the postnatal period. To determine how the number of myonuclei changes during this period of intense muscle fibre hypertrophy, we used two complementary mouse models: 3F-nlacZ-E mice express nlacZ only in myonuclei, while Myf5nlacZ/+ mice have beta-galactosidase activity in satellite cells. There was a approximately 5-fold increase in myonuclear number per myofibre between P3 and P21. Thus myofibre hypertrophy is initially accompanied by a significant addition of myonuclei. Despite this, the estimated myonuclear domain still doubled between P7 and P21 to 9.2 x 103 microm3. There was no further addition of myonuclei from P21, but myofibre volume continued to increase, resulting in an estimated approximately 3-fold expansion of the myonuclear domain to 26.5 x 103 microm3 by P56. We also used our two mouse models to determine the number of satellite cells per myofibre during postnatal growth. Satellite cell number in EDL was initially approximately 14 satellite cells per myofibre at P7, but then fell to reach the adult level of approximately 5 by P21.
Conclusions: Postnatal fast muscle fibre type growth is divided into distinct phases in mouse EDL: myofibre hypertrophy is initially supported by a rapid increase in the number of myonuclei, but nuclear addition stops around P21. Since the significant myofibre hypertrophy from P21 to adulthood occurs without the net addition of new myonuclei, a considerable expansion of the myonuclear domain results. Satellite cell numbers are initially stable, but then decrease to reach the adult level by P21. Thus the adult number of both myonuclei and satellite cells is already established by three weeks of postnatal growth in mouse.
Figures

References
-
- Ross JJ, Duxson MJ, Harris AJ. Formation of primary and secondary myotubes in rat lumbrical muscles. Development. 1987;100:383–94. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

