Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes
- PMID: 20175925
- PMCID: PMC2838829
- DOI: 10.1186/1471-2121-11-15
Green-to-red photoconvertible fluorescent proteins: tracking cell and protein dynamics on standard wide-field mercury arc-based microscopes
Abstract
Background: Green fluorescent protein (GFP) and other FP fusions have been extensively utilized to track protein dynamics in living cells. Recently, development of photoactivatable, photoswitchable and photoconvertible fluorescent proteins (PAFPs) has made it possible to investigate the fate of discrete subpopulations of tagged proteins. Initial limitations to their use (due to their tetrameric nature) were overcome when monomeric variants, such as Dendra, mEos, and mKikGR were cloned/engineered.
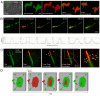
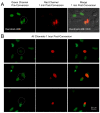
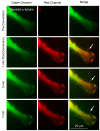
Results: Here, we report that by closing the field diaphragm, selective, precise and irreversible green-to-red photoconversion (330-380 nm illumination) of discrete subcellular protein pools was achieved on a wide-field fluorescence microscope equipped with standard DAPI, Fluorescein, and Rhodamine filter sets and mercury arc illumination within 5-10 seconds. Use of a DAPI-filter cube with long-pass emission filter (LP420) allowed the observation and control of the photoconversion process in real time. Following photoconversion, living cells were imaged for up to 5 hours often without detectable phototoxicity or photobleaching.
Conclusions: We demonstrate the practicability of this technique using Dendra2 and mEos2 as monomeric, photoconvertible PAFP representatives fused to proteins with low (histone H2B), medium (gap junction channel protein connexin 43), and high (alpha-tubulin; clathrin light chain) dynamic cellular mobility as examples. Comparable efficient, irreversible green-to-red photoconversion of selected portions of cell nuclei, gap junctions, microtubules and clathrin-coated vesicles was achieved. Tracking over time allowed elucidation of the dynamic live-cycle of these subcellular structures. The advantage of this technique is that it can be performed on a standard, relatively inexpensive wide-field fluorescence microscope with mercury arc illumination. Together with previously described laser scanning confocal microscope-based photoconversion methods, this technique promises to further increase the general usability of photoconvertible PAFPs to track the dynamic movement of cells and proteins over time.
Figures

Similar articles
-
Exploring plant endomembrane dynamics using the photoconvertible protein Kaede.Plant J. 2010 Aug;63(4):696-711. doi: 10.1111/j.1365-313X.2010.04272.x. Plant J. 2010. PMID: 20545892
-
Using photoactivatable fluorescent protein Dendra2 to track protein movement.Biotechniques. 2007 May;42(5):553, 555, 557 passim. doi: 10.2144/000112470. Biotechniques. 2007. PMID: 17515192
-
Color recovery after photoconversion of H2B::mEosFP allows detection of increased nuclear DNA content in developing plant cells.Plant Physiol. 2012 Jan;158(1):95-106. doi: 10.1104/pp.111.187062. Epub 2011 Nov 22. Plant Physiol. 2012. PMID: 22108524 Free PMC article.
-
High resolution, fluorescence deconvolution microscopy and tagging with the autofluorescent tracers CFP, GFP, and YFP to study the structural composition of gap junctions in living cells.Microsc Res Tech. 2001 Feb 1;52(3):251-62. doi: 10.1002/1097-0029(20010201)52:3<251::AID-JEMT1011>3.0.CO;2-#. Microsc Res Tech. 2001. PMID: 11180618 Review.
-
From fixed to FRAP: measuring protein mobility and activity in living cells.Nat Cell Biol. 2001 Jun;3(6):E145-7. doi: 10.1038/35078615. Nat Cell Biol. 2001. PMID: 11389456 Review.
Cited by
-
Finding and tracing human MSC in 3D microenvironments with the photoconvertible protein Dendra2.Sci Rep. 2015 May 14;5:10079. doi: 10.1038/srep10079. Sci Rep. 2015. PMID: 25974085 Free PMC article.
-
PIN2 turnover in Arabidopsis root epidermal cells explored by the photoconvertible protein Dendra2.PLoS One. 2013 Apr 18;8(4):e61403. doi: 10.1371/journal.pone.0061403. Print 2013. PLoS One. 2013. PMID: 23637828 Free PMC article.
-
Deubiquitinase dynamics: methodologies for understanding substrate interactions.BMB Rep. 2025 May;58(5):191-202. doi: 10.5483/BMBRep.2025-0011. BMB Rep. 2025. PMID: 40058876 Free PMC article. Review.
-
Correlated light and electron microscopy: ultrastructure lights up!Nat Methods. 2015 Jun;12(6):503-13. doi: 10.1038/nmeth.3400. Nat Methods. 2015. PMID: 26020503 Review.
-
Cell tracking using photoconvertible proteins during zebrafish development.J Vis Exp. 2012 Sep 28;(67):4350. doi: 10.3791/4350. J Vis Exp. 2012. PMID: 23052298 Free PMC article.
References
-
- Piston DW, Kremers G-J, Benninger RKP, Davidson MW. Photoactivation in fluorescence microscopy. Microscopy Today. 2009;17(4):8–13. doi: 10.1017/S1551929509000194. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

