Redox-optimized ROS balance: a unifying hypothesis
- PMID: 20175987
- PMCID: PMC2891851
- DOI: 10.1016/j.bbabio.2010.02.016
Redox-optimized ROS balance: a unifying hypothesis
Abstract
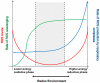
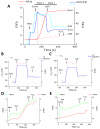
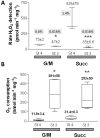
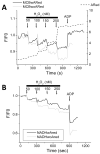
While it is generally accepted that mitochondrial reactive oxygen species (ROS) balance depends on the both rate of single electron reduction of O2 to superoxide (O2.-) by the electron transport chain and the rate of scavenging by intracellular antioxidant pathways, considerable controversy exists regarding the conditions leading to oxidative stress in intact cells versus isolated mitochondria. Here, we postulate that mitochondria have been evolutionarily optimized to maximize energy output while keeping ROS overflow to a minimum by operating in an intermediate redox state. We show that at the extremes of reduction or oxidation of the redox couples involved in electron transport (NADH/NAD+) or ROS scavenging (NADPH/NADP+, GSH/GSSG), respectively, ROS balance is lost. This results in a net overflow of ROS that increases as one moves farther away from the optimal redox potential. At more reduced mitochondrial redox potentials, ROS production exceeds scavenging, while under more oxidizing conditions (e.g., at higher workloads) antioxidant defenses can be compromised and eventually overwhelmed. Experimental support for this hypothesis is provided in both cardiomyocytes and in isolated mitochondria from guinea pig hearts. The model reconciles, within a single framework, observations that isolated mitochondria tend to display increased oxidative stress at high reduction potentials (and high mitochondrial membrane potential, Psim), whereas intact cardiac cells can display oxidative stress either when mitochondria become more uncoupled (i.e., low Psim) or when mitochondria are maximally reduced (as in ischemia or hypoxia). The continuum described by the model has the potential to account for many disparate experimental observations and also provides a rationale for graded physiological ROS signaling at redox potentials near the minimum.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures






References
-
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. - PubMed
-
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. - PubMed
-
- Pearl R. The Rate of Living. University of London Press; London: 1928.
-
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

