Regulatory activity of polyunsaturated fatty acids in T-cell signaling
- PMID: 20176053
- PMCID: PMC2872685
- DOI: 10.1016/j.plipres.2010.01.002
Regulatory activity of polyunsaturated fatty acids in T-cell signaling
Abstract
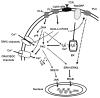
n-3 Polyunsaturated fatty acids (PUFA) are considered to be authentic immunosuppressors and appear to exert beneficial effects with respect to certain immune-mediated diseases. In addition to promoting T-helper 1 (Th1) cell to T-helper 2 (Th2) cell effector T-cell differentiation, n-3 PUFA may also exert anti-inflammatory actions by inducing apoptosis in Th1 cells. With respect to mechanisms of action, effects range from the modulation of membrane receptors to gene transcription via perturbation of a number of second messenger cascades. In this review, the putative targets of anti-inflammatory n-3 PUFA, activated during early and late events of T-cell activation will be discussed. Studies have demonstrated that these fatty acids alter plasma membrane micro-organization (lipid rafts) at the immunological synapse, the site where T-cells and antigen-presenting cells (APC) form a physical contact for antigen initiated T-cell signaling. In addition, the production of diacylglycerol and the activation of different isoforms of protein kinase C (PKC), mitogen-activated protein kinase (MAPK), calcium signaling, and nuclear translocation/activation of transcriptional factors, can be modulated by n-3 PUFA. Advantages and limitations of diverse methodologies to study the membrane lipid raft hypothesis, as well as apparent contradictions regarding the effect of n-3 PUFA on lipid rafts will be critically presented.
Figures


Similar articles
-
Polyunsaturated fatty acids in the modulation of T-cell signalling.Prostaglandins Leukot Essent Fatty Acids. 2010 Apr-Jun;82(4-6):179-87. doi: 10.1016/j.plefa.2010.02.023. Epub 2010 Mar 1. Prostaglandins Leukot Essent Fatty Acids. 2010. PMID: 20189788 Review.
-
Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production.J Immunol. 2004 Nov 15;173(10):6151-60. doi: 10.4049/jimmunol.173.10.6151. J Immunol. 2004. PMID: 15528352
-
Polyunsaturated fatty acids interfere with formation of the immunological synapse.J Leukoc Biol. 2005 May;77(5):680-8. doi: 10.1189/jlb.1104687. Epub 2005 Feb 9. J Leukoc Biol. 2005. PMID: 15703198
-
Dietary (n-3) polyunsaturated fatty acids remodel mouse T-cell lipid rafts.J Nutr. 2003 Jun;133(6):1913-20. doi: 10.1093/jn/133.6.1913. J Nutr. 2003. PMID: 12771339
-
Membrane lipid rafts: new targets for immunoregulation.Curr Mol Med. 2002 Sep;2(6):557-70. doi: 10.2174/1566524023362122. Curr Mol Med. 2002. PMID: 12243248 Review.
Cited by
-
N-3 fatty acids and membrane microdomains: from model membranes to lymphocyte function.Prostaglandins Leukot Essent Fatty Acids. 2012 Dec;87(6):205-8. doi: 10.1016/j.plefa.2012.09.007. Epub 2012 Oct 27. Prostaglandins Leukot Essent Fatty Acids. 2012. PMID: 23107229 Free PMC article. Review.
-
Recent advances in the field of nutritional immunology.Expert Rev Clin Immunol. 2011 Nov;7(6):747-9. doi: 10.1586/eci.11.69. Expert Rev Clin Immunol. 2011. PMID: 22014015 Free PMC article. Review.
-
Maternal diet supplementation with high-docosahexaenoic-acid canola oil, along with arachidonic acid, promotes immune system development in allergy-prone BALB/c mouse offspring at 3 weeks of age.Eur J Nutr. 2023 Sep;62(6):2399-2413. doi: 10.1007/s00394-023-03160-6. Epub 2023 Apr 27. Eur J Nutr. 2023. PMID: 37106253
-
Th17 cell accumulation is decreased during chronic experimental colitis by (n-3) PUFA in Fat-1 mice.J Nutr. 2012 Jan;142(1):117-24. doi: 10.3945/jn.111.147058. Epub 2011 Nov 30. J Nutr. 2012. PMID: 22131549 Free PMC article.
-
Can N-3 polyunsaturated fatty acids be considered a potential adjuvant therapy for COVID-19-associated cardiovascular complications?Pharmacol Ther. 2021 Mar;219:107703. doi: 10.1016/j.pharmthera.2020.107703. Epub 2020 Oct 5. Pharmacol Ther. 2021. PMID: 33031856 Free PMC article. Review.
References
-
- Chapkin RS. Reappraisal of the essential fatty acids. In: Chow CK, editor. Fatty acids in foods and their health implications. 3rd. CRC Press; New York: 2008. pp. 675–691.
-
- Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323:645–655. - PubMed
-
- Lands WE. Biosynthesis of prostaglandins. Annu Rev Nutr. 1991;11:41–60. - PubMed
-
- Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. - PubMed
-
- Calder PC. Dietary modification of inflammation with lipids. Proc Nutr Soc. 2002;61:345–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

