Relevance of gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) in the structural integrity of the chromatoid body during spermatogenesis
- PMID: 20176059
- PMCID: PMC2856730
- DOI: 10.1016/j.bbamcr.2010.02.004
Relevance of gonadotropin-regulated testicular RNA helicase (GRTH/DDX25) in the structural integrity of the chromatoid body during spermatogenesis
Abstract
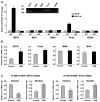
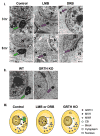
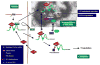
Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25), a multifunctional protein and a component of ribonucleoprotein complexes, is essential for the completion of spermatogenesis. We investigated the nuclear/cytoplasmic shuttling of GRTH in germ cells and its impact on the chromatoid body (CB)-a perinuclear organelle viewed as a storage/processing site of mRNAs. GRTH resides in the nucleus, cytoplasm and CB of round spermatids. Treatment of these cells with inhibitors of nuclear export or RNA synthesis caused nuclear retention of GRTH and its absence in the cytoplasm and CB. The nuclear levels of GRTH bound RNA messages were significantly enhanced and major reduction was observed in the cytoplasm. This indicated GRTH main transport function of mRNAs to the cytoplasm and CB. MVH, a germ cell helicase, and MIWI, a component of the RNA-induced-silencing complex (RISC), confined to the CB/cytoplasm, were absent in the CB and accumulated in the cytoplasm upon treatment. This also occurred in spermatids of GRTH-KO mice. The CB changed from lobular-filamentous to a small condensed structure after treatment resembling the CB of GRTH-KO. No interaction of GRTH with MVH or RISC members in both protein and RNA were observed. Besides of participating in the transport of messages of relevant spermatogenic genes, GRTH was found to transport its own message to cytoplasmic sites. Our studies suggest that GRTH through its export/transport function as a component of mRNP is essential to govern the CB structure in spermatids and to maintain systems that may participate in mRNA storage and their processing during spermatogenesis.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures



References
-
- Steger K. Haploid spermatids exhibit translationally repressed mRNAs. Anat Embryol (Berl) 2001;203:323–334. - PubMed
-
- Toppari J, Kangasniemi M, Kaipia A, Mali P, Huhtaniemi I, Parvinen M. Stage- and cell-specific gene expression and hormone regulation of the seminiferous epithelium. J Electron Microsc Tech. 1991;19:203–214. - PubMed
-
- Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat Rev Mol Cell Biol. 2007;8:9–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

