Reserpine-induced reduction in norepinephrine transporter function requires catecholamine storage vesicles
- PMID: 20176067
- PMCID: PMC2859979
- DOI: 10.1016/j.neuint.2010.02.011
Reserpine-induced reduction in norepinephrine transporter function requires catecholamine storage vesicles
Abstract
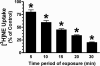
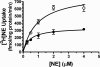
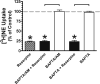
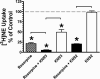
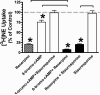
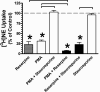
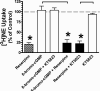
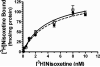
Treatment of rats with reserpine, an inhibitor of the vesicular monoamine transporter (VMAT), depletes norepinephrine (NE) and regulates NE transporter (NET) expression. The present study examined the molecular mechanisms involved in regulation of the NET by reserpine using cultured cells. Exposure of rat PC12 cells to reserpine for a period as short as 5min decreased [(3)H]NE uptake capacity, an effect characterized by a robust decrease in the V(max) of the transport of [(3)H]NE. As expected, reserpine did not displace the binding of [(3)H]nisoxetine from the NET in membrane homogenates. The potency of reserpine for reducing [(3)H]NE uptake was dramatically lower in SK-N-SH cells that have reduced storage capacity for catecholamines. Reserpine had no effect on [(3)H]NE uptake in HEK-293 cells transfected with the rat NET (293-hNET), cells that lack catecholamine storage vesicles. NET regulation by reserpine was independent of trafficking of the NET from the cell surface. Pre-exposure of cells to inhibitors of several intracellular signaling cascades known to regulate the NET, including Ca(2+)/Ca(2+)-calmodulin dependent kinase and protein kinases A, C and G, did not affect the ability of reserpine to reduce [(3)H]NE uptake. Treatment of PC12 cells with the catecholamine depleting agent, alpha-methyl-p-tyrosine, increased [(3)H]NE uptake and eliminated the inhibitory effects of reserpine on [(3)H]NE uptake. Reserpine non-competitively inhibits NET activity through a Ca(2+)-independent process that requires catecholamine storage vesicles, revealing a novel pharmacological method to modify NET function. Further characterization of the molecular nature of reserpine's action could lead to the development of alternative therapeutic strategies for treating disorders known to be benefitted by treatment with traditional competitive NET inhibitors.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures

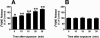
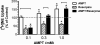
Similar articles
-
Depletion of cardiac catecholamine stores impairs cardiac norepinephrine re-uptake by downregulation of the norepinephrine transporter.PLoS One. 2017 Mar 10;12(3):e0172070. doi: 10.1371/journal.pone.0172070. eCollection 2017. PLoS One. 2017. PMID: 28282374 Free PMC article.
-
Vesicular monoamine transport inhibitors. Novel action at calcium channels to prevent catecholamine secretion.Hypertension. 1996 Sep;28(3):414-20. doi: 10.1161/01.hyp.28.3.414. Hypertension. 1996. PMID: 8794826
-
Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells.J Pharmacol Exp Ther. 2001 Nov;299(2):666-77. J Pharmacol Exp Ther. 2001. PMID: 11602680
-
Pentazocine inhibits norepinephrine transporter function by reducing its surface expression in bovine adrenal medullary cells.J Pharmacol Sci. 2013;121(2):138-47. doi: 10.1254/jphs.12164fp. Epub 2013 Feb 1. J Pharmacol Sci. 2013. PMID: 23370666
-
[11C]-p-Hydroxyphenethylguanidine.2007 Jan 18 [updated 2008 Feb 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2007 Jan 18 [updated 2008 Feb 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641521 Free Books & Documents. Review.
Cited by
-
Depletion of cardiac catecholamine stores impairs cardiac norepinephrine re-uptake by downregulation of the norepinephrine transporter.PLoS One. 2017 Mar 10;12(3):e0172070. doi: 10.1371/journal.pone.0172070. eCollection 2017. PLoS One. 2017. PMID: 28282374 Free PMC article.
-
Indole-containing pharmaceuticals: targets, pharmacological activities, and SAR studies.RSC Med Chem. 2024 Jan 30;15(3):788-808. doi: 10.1039/d3md00677h. eCollection 2024 Mar 20. RSC Med Chem. 2024. PMID: 38516587 Free PMC article. Review.
-
Dengue Virus Replication Is Associated with Catecholamine Biosynthesis and Metabolism in Hepatocytes.Viruses. 2022 Mar 9;14(3):564. doi: 10.3390/v14030564. Viruses. 2022. PMID: 35336971 Free PMC article.
-
Rationalizing the Binding Modes of PET Radiotracers Targeting the Norepinephrine Transporter.Pharmaceutics. 2023 Feb 17;15(2):690. doi: 10.3390/pharmaceutics15020690. Pharmaceutics. 2023. PMID: 36840011 Free PMC article.
-
Association of Hepatitis C Virus Replication with the Catecholamine Biosynthetic Pathway.Viruses. 2021 Oct 23;13(11):2139. doi: 10.3390/v13112139. Viruses. 2021. PMID: 34834946 Free PMC article.
References
-
- Apparsundaram S, Galli A, DeFelice LJ, Hartzell HC, Blakely RD. Acute regulation of norepinephrine transport: I. protein kinase C-linked muscarinic receptors influence transport capacity and transporter density in SK-N-SH cells. J. Pharmacol. Exp. Ther. 1998a;287:733–43. - PubMed
-
- Apparsundaram S, Schroeter S, Giovanetti E, Blakely RD. Acute regulation of norepinephrine transport: II. PKC-modulated surface expression of human norepinephrine transporter proteins. J. Pharmacol. Exp. Ther. 1998b;287:744–51. - PubMed
-
- Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and - independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J. Pharmacol. Exp. Ther. 2001;299:666–77. - PubMed
-
- Benmansour S, Altamirano AV, Jones DJ, Sanchez TA, Gould GG, Pardon MC, Morilak DA, Frazer A. Regulation of the norepinephrine transporter by chronic administration of antidepressants. Biol. Psychiatry. 2004;55:313–6. - PubMed
-
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

