Purification and functional characterization of human mitochondrial DNA polymerase gamma harboring disease mutations
- PMID: 20176107
- PMCID: PMC2901396
- DOI: 10.1016/j.ymeth.2010.02.015
Purification and functional characterization of human mitochondrial DNA polymerase gamma harboring disease mutations
Abstract
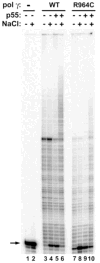
More than 150 different point mutations in POLG, the gene encoding the human mitochondrial DNA polymerase gamma (pol gamma), cause a broad spectrum of childhood and adult onset diseases like Alpers syndrome, ataxia-neuropathy syndrome and progressive external ophthalmoplegia. These disease mutations can affect the pol gamma enzyme's properties in numerous ways, thus potentially influencing the severity of the disease. Hence, a detailed characterization of disease mutants will greatly assist researchers and clinicians to develop a clear understanding of the functional defects caused by these mutant enzymes. Experimental approaches for characterizing the wild-type (WT) and mutant pol gamma enzymes are extensively described in this manuscript. The methods start with construction and purification of the recombinant wild-type and mutant forms of pol gamma protein, followed by assays to determine its structural integrity and thermal stability. Next, the biochemical characterization of these enzymes is described in detail, which includes measuring the purified enzyme's catalytic activity, its steady-state kinetic parameters and DNA binding activity, and determining the physical and functional interaction of these pol gamma proteins with the p55 accessory subunit.
Published by Elsevier Inc.
Figures


Similar articles
-
Synergistic Effects of the in cis T251I and P587L Mitochondrial DNA Polymerase γ Disease Mutations.J Biol Chem. 2017 Mar 10;292(10):4198-4209. doi: 10.1074/jbc.M116.773341. Epub 2017 Feb 2. J Biol Chem. 2017. PMID: 28154168 Free PMC article.
-
Functional analysis of mutant mitochondrial DNA polymerase proteins involved in human disease.Methods Mol Biol. 2009;554:59-72. doi: 10.1007/978-1-59745-521-3_4. Methods Mol Biol. 2009. PMID: 19513667 Free PMC article.
-
Disease mutations in the human mitochondrial DNA polymerase thumb subdomain impart severe defects in mitochondrial DNA replication.J Biol Chem. 2009 Jul 17;284(29):19501-10. doi: 10.1074/jbc.M109.011940. Epub 2009 May 28. J Biol Chem. 2009. PMID: 19478085 Free PMC article.
-
Mitochondrial DNA replication and disease: insights from DNA polymerase γ mutations.Cell Mol Life Sci. 2011 Jan;68(2):219-33. doi: 10.1007/s00018-010-0530-4. Epub 2010 Oct 8. Cell Mol Life Sci. 2011. PMID: 20927567 Free PMC article. Review.
-
Clinical and molecular features of POLG-related mitochondrial disease.Cold Spring Harb Perspect Biol. 2013 Apr 1;5(4):a011395. doi: 10.1101/cshperspect.a011395. Cold Spring Harb Perspect Biol. 2013. PMID: 23545419 Free PMC article. Review.
Cited by
-
Mutations in human DNA polymerase γ confer unique mechanisms of catalytic deficiency that mirror the disease severity in mitochondrial disorder patients.Hum Mol Genet. 2013 Mar 15;22(6):1074-85. doi: 10.1093/hmg/dds509. Epub 2012 Dec 3. Hum Mol Genet. 2013. PMID: 23208208 Free PMC article.
-
Human mitochondrial DNA polymerase γ exhibits potential for bypass and mutagenesis at UV-induced cyclobutane thymine dimers.J Biol Chem. 2012 Mar 16;287(12):9222-9. doi: 10.1074/jbc.M111.306852. Epub 2011 Dec 21. J Biol Chem. 2012. PMID: 22194617 Free PMC article.
-
Characterization of the human homozygous R182W POLG2 mutation in mitochondrial DNA depletion syndrome.PLoS One. 2018 Aug 29;13(8):e0203198. doi: 10.1371/journal.pone.0203198. eCollection 2018. PLoS One. 2018. PMID: 30157269 Free PMC article.
-
Structure-specific roles for PolG2-DNA complexes in maintenance and replication of mitochondrial DNA.Nucleic Acids Res. 2023 Oct 13;51(18):9716-9732. doi: 10.1093/nar/gkad679. Nucleic Acids Res. 2023. PMID: 37592734 Free PMC article.
-
Synergistic Effects of the in cis T251I and P587L Mitochondrial DNA Polymerase γ Disease Mutations.J Biol Chem. 2017 Mar 10;292(10):4198-4209. doi: 10.1074/jbc.M116.773341. Epub 2017 Feb 2. J Biol Chem. 2017. PMID: 28154168 Free PMC article.
References
-
- Bebenek K, Kunkel TA. Adv Protein Chem. 2004;69:137–65. - PubMed
-
- Ropp PA, Copeland WC. Genomics. 1996;36:449–58. - PubMed
-
- Sweasy JB, Lauper JM, Eckert KA. Radiat Res. 2006;166:693–714. - PubMed
-
- Graziewicz MA, Longley MJ, Copeland WC. Chemical Reviews. 2006;106:383–405. - PubMed
-
- Lim SE, Longley MJ, Copeland WC. J Biol Chem. 1999;274:38197–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

