Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease
- PMID: 20176130
- PMCID: PMC2886168
- DOI: 10.1016/j.bbalip.2010.02.005
Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease
Abstract
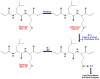
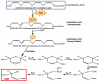
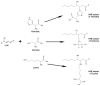
Alzheimer's disease (AD) is an age-related neurodegenerative disorder. A number of hypotheses have been proposed to explain AD pathogenesis. One such hypothesis proposed to explain AD pathogenesis is the oxidative stress hypothesis. Increased levels of oxidative stress markers including the markers of lipid peroxidation such as acrolein, 4-hydroxy-2-trans-nonenal (HNE), malondialdehyde, etc. are found in brains of AD subjects. In this review, we focus principally on research conducted in the area of HNE in the central nervous system (CNS) of AD and mild cognitive impairment (MCI), and further, we discuss likely consequences of lipid peroxidation with respect to AD pathogenesis and progression. Based on the research conducted so far in the area of lipid peroxidation, it is suggested that lipid accessible antioxidant molecules could be a promising therapeutic approach to treat or slow progression of MCI and AD.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures
References
-
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. - PubMed
-
- Butterfield DA, Stadtman ER. Protein Oxidation processes in aging brain. Adv Cell Aging Gerontol. 1997;2:161–191.
-
- Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free radical biology & medicine. 1997;23:134–147. - PubMed
-
- Nunomura A, Moreira PI, Lee HG, Zhu X, Castellani RJ, Smith MA, Perry G. Neuronal death and survival under oxidative stress in Alzheimer and Parkinson diseases. CNS Neurol Disord Drug Targets. 2007;6:411–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

