Manganese upregulates cellular prion protein and contributes to altered stabilization and proteolysis: relevance to role of metals in pathogenesis of prion disease
- PMID: 20176619
- PMCID: PMC2871751
- DOI: 10.1093/toxsci/kfq049
Manganese upregulates cellular prion protein and contributes to altered stabilization and proteolysis: relevance to role of metals in pathogenesis of prion disease
Abstract
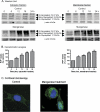
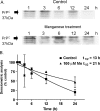
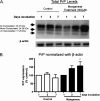
Prion diseases are fatal neurodegenerative diseases resulting from misfolding of normal cellular prion (PrP(C)) into an abnormal form of scrapie prion (PrP(Sc)). The cellular mechanisms underlying the misfolding of PrP(C) are not well understood. Since cellular prion proteins harbor divalent metal-binding sites in the N-terminal region, we examined the effect of manganese on PrP(C) processing in in vitro models of prion disease. Exposure to manganese significantly increased PrP(C) levels both in cytosolic and in membrane-rich fractions in a time-dependent manner. Manganese-induced PrP(C) upregulation was independent of messenger RNA transcription or stability. Additionally, manganese treatment did not alter the PrP(C) degradation by either proteasomal or lysosomal pathways. Interestingly, pulse-chase analysis showed that the PrP(C) turnover rate was significantly altered with manganese treatment, indicating increased stability of PrP(C) with the metal exposure. Limited proteolysis studies with proteinase-K further supported that manganese increases the stability of PrP(C). Incubation of mouse brain slice cultures with manganese also resulted in increased prion protein levels and higher intracellular manganese accumulation. Furthermore, exposure of manganese to an infectious prion cell model, mouse Rocky Mountain Laboratory-infected CAD5 cells, significantly increased prion protein levels. Collectively, our results demonstrate for the first time that divalent metal manganese can alter the stability of prion proteins and suggest that manganese-induced stabilization of prion protein may play a role in prion protein misfolding and prion disease pathogenesis.
Figures


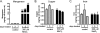

Similar articles
-
Modulation of proteinase K-resistant prion protein in cells and infectious brain homogenate by redox iron: implications for prion replication and disease pathogenesis.Mol Biol Cell. 2007 Sep;18(9):3302-12. doi: 10.1091/mbc.e07-04-0317. Epub 2007 Jun 13. Mol Biol Cell. 2007. PMID: 17567949 Free PMC article.
-
Infectious prion protein alters manganese transport and neurotoxicity in a cell culture model of prion disease.Neurotoxicology. 2011 Oct;32(5):554-62. doi: 10.1016/j.neuro.2011.07.008. Epub 2011 Aug 19. Neurotoxicology. 2011. PMID: 21871919 Free PMC article.
-
In vitro amplification of scrapie and chronic wasting disease PrP(res) using baculovirus-expressed recombinant PrP as substrate.Prion. 2014;8(6):393-403. doi: 10.4161/19336896.2014.983753. Prion. 2014. PMID: 25495764 Free PMC article.
-
Neurometals in the Pathogenesis of Prion Diseases.Int J Mol Sci. 2021 Jan 28;22(3):1267. doi: 10.3390/ijms22031267. Int J Mol Sci. 2021. PMID: 33525334 Free PMC article. Review.
-
Interaction of metals with prion protein: possible role of divalent cations in the pathogenesis of prion diseases.Neurotoxicology. 2006 Sep;27(5):777-87. doi: 10.1016/j.neuro.2006.06.004. Epub 2006 Jun 18. Neurotoxicology. 2006. PMID: 16860868 Review.
Cited by
-
Up-regulation of mRNA ventricular PRNP prion protein gene expression in air pollution highly exposed young urbanites: endoplasmic reticulum stress, glucose regulated protein 78, and nanosized particles.Int J Mol Sci. 2013 Nov 28;14(12):23471-91. doi: 10.3390/ijms141223471. Int J Mol Sci. 2013. PMID: 24287918 Free PMC article.
-
Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation.Front Neurosci. 2019 Jun 26;13:654. doi: 10.3389/fnins.2019.00654. eCollection 2019. Front Neurosci. 2019. PMID: 31293375 Free PMC article. Review.
-
Metals and Neurodegeneration.F1000Res. 2016 Mar 17;5:F1000 Faculty Rev-366. doi: 10.12688/f1000research.7431.1. eCollection 2016. F1000Res. 2016. PMID: 27006759 Free PMC article. Review.
-
Biomarkers of manganese intoxication.Neurotoxicology. 2011 Jan;32(1):1-8. doi: 10.1016/j.neuro.2010.10.002. Epub 2010 Oct 12. Neurotoxicology. 2011. PMID: 20946915 Free PMC article. Review.
-
Neurodegenerative Proteinopathies Induced by Environmental Pollutants: Heat Shock Proteins and Proteasome as Promising Therapeutic Tools.Pharmaceutics. 2023 Jul 30;15(8):2048. doi: 10.3390/pharmaceutics15082048. Pharmaceutics. 2023. PMID: 37631262 Free PMC article. Review.
References
-
- Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat. Rev. Microbiol. 2006;4:765–775. - PubMed
-
- Bocharova OV, Breydo L, Salnikov VV, Baskakov IV. Copper(II) inhibits in vitro conversion of prion protein into amyloid fibrils. Biochemistry. 2005;44:6776–6787. - PubMed
-
- Brazier MW, Davies P, Player E, Marken F, Viles JH, Brown DR. Manganese binding to the prion protein. J. Biol. Chem. 2008;283:12831–12839. - PubMed
-
- Brown DR. Brain proteins that mind metals: a neurodegenerative perspective. Dalton Trans. 2009;21:4069–4076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

