Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability
- PMID: 20176628
- PMCID: PMC2872738
- DOI: 10.1113/jphysiol.2009.184168
Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability
Abstract
Decreased maternal nutrient availability during pregnancy induces compensatory fetal metabolic and endocrine responses. Knowledge of cellular changes involved is critical to understanding normal and abnormal development. Several studies in rodents and sheep report increased fetal plasma cortisol and associated increased gluconeogenesis in response to maternal nutrient reduction (MNR) but observations in primates are lacking. We determined MNR effects on fetal liver phosphoenolpyruvate carboxykinase 1 (protein, PEPCK1; gene, PCK1 orthologous/homologous human chromosomal region 20q13.31) at 0.9 gestation (G). Female baboon social groups were fed ad libitum (control, CTR) or 70% CTR (MNR) from 0.16 to 0.9G when fetuses were delivered by caesarean section under general anaesthesia. Plasma cortisol was elevated in fetuses of MNR mothers (P < 0.05). Immunoreactive PEPCK1 protein was located around the liver lobule central vein and was low in CTR fetuses but rose to 63% of adult levels in MNR fetuses. PCK1 mRNA measured by QRT-PCR increased in MNR (2.3-fold; P < 0.05) while the 25% rise in protein by Western blot analysis was not significant. PCK1 promoter methylation analysis using bisulfite sequencing was significantly reduced in six out of nine CpG-dinucleotides evaluated in MNR compared with CTR liver samples. In conclusion, these are the first data from a fetal non-human primate indicating hypomethylation of the PCK1 promoter in the liver following moderate maternal nutrient reduction.
Figures
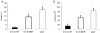

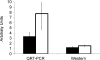

Comment in
-
Remembering development - epigenetic responses to fetal malnutrition.J Physiol. 2010 May 1;588(Pt 9):1379-80. doi: 10.1113/jphysiol.2010.189787. J Physiol. 2010. PMID: 20436037 Free PMC article. No abstract available.
References
-
- Bock C, Reither S, Mikeska T, Paulsen M, Walter J, Lengauer T. BiQ Analyzer: visualization and quality control for DNA methylation data from bisulfite sequencing. Bioinformatics. 2005;21:4067–4068. - PubMed
-
- Cassuto H, Kochan K, Chakravarty K, Cohen H, Blum B, Olswang Y, Hakimi P, Xu C, Massillon D, Hanson RW, Reshef L. Glucocorticoids regulate transcription of the gene for phosphoenolpyruvate carboxykinase in the liver via an extended glucocorticoid regulatory unit. J Biol Chem. 2005;280:33873–33884. - PubMed
-
- Chakravarty K, Cassuto H, Reshef L, Hanson RW. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit Rev Biochem Mol Biol. 2005;40:129–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

