Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors
- PMID: 20176630
- PMCID: PMC2872731
- DOI: 10.1113/jphysiol.2009.182444
Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors
Abstract
The activation characteristics of synaptic and extrasynaptic GABA(A) receptors are important for shaping the profile of phasic and tonic inhibition in the central nervous system, which will critically impact on the activity of neuronal networks. Here, we study in isolation the activity of three agonists, GABA, muscimol and 4,5,6,7-tetrahydoisoxazolo[5,4-c]pyridin-3(2H)-one (THIP), to further understand the activation profiles of alpha 1 beta 3 gamma 2, alpha 4 beta 3 gamma 2 and alpha 4 beta 3 delta receptors that typify synaptic- and extrasynaptic-type receptors expressed in the hippocampus and thalamus. The agonists display an order of potency that is invariant between the three receptors, which is reliant mostly on the agonist dissociation constant. At delta subunit-containing extrasynaptic-type GABA(A) receptors, both THIP and muscimol additionally exhibited, to different degrees, superagonist behaviour. By comparing whole-cell and single channel currents induced by the agonists, we provide a molecular explanation for their different activation profiles. For THIP at high concentrations, the unusual superagonist behaviour on alpha 4 beta 3 delta receptors is a consequence of its ability to increase the duration of longer channel openings and their frequency, resulting in longer burst durations. By contrast, for muscimol, moderate superagonist behaviour was caused by reduced desensitisation of the extrasynaptic-type receptors. The ability to specifically increase the efficacy of receptor activation, by selected exogenous agonists over that obtained with the natural transmitter, may prove to be of therapeutic benefit under circumstances when synaptic inhibition is compromised or dysfunctional.
Figures




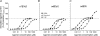
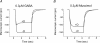
Comment in
-
Pharmacological studies reveal novel aspects of the versatility of GABAA receptors.J Physiol. 2010 May 1;588(Pt 9):1381-2. doi: 10.1113/jphysiol.2010.189910. J Physiol. 2010. PMID: 20436038 Free PMC article. No abstract available.
Similar articles
-
Agonist-dependent single channel current and gating in alpha4beta2delta and alpha1beta2gamma2S GABAA receptors.J Gen Physiol. 2008 Feb;131(2):163-81. doi: 10.1085/jgp.200709871. J Gen Physiol. 2008. PMID: 18227274 Free PMC article.
-
Extrasynaptic GABAA receptors in rat pontine reticular formation increase wakefulness.Sleep. 2013 Mar 1;36(3):337-43. doi: 10.5665/sleep.2444. Sleep. 2013. PMID: 23450652 Free PMC article.
-
Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex.J Neurophysiol. 2007 Mar;97(3):2293-300. doi: 10.1152/jn.00651.2006. Epub 2007 Jan 10. J Neurophysiol. 2007. PMID: 17215511
-
Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs.J Clin Sleep Med. 2006 Apr 15;2(2):S12-8. J Clin Sleep Med. 2006. PMID: 17557502 Review.
-
Mechanisms of sleep induction by GABA(A) receptor agonists.J Clin Psychiatry. 2007;68 Suppl 5:6-12. J Clin Psychiatry. 2007. PMID: 17539703 Review.
Cited by
-
Lack of an endogenous GABAA receptor-mediated tonic current in hypoglossal motoneurons.J Physiol. 2012 Jul 1;590(13):2965-76. doi: 10.1113/jphysiol.2012.231944. Epub 2012 Apr 10. J Physiol. 2012. PMID: 22495589 Free PMC article.
-
Conformational state-dependent regulation of GABAA receptor diffusion and subsynaptic domains.iScience. 2022 Oct 29;25(11):105467. doi: 10.1016/j.isci.2022.105467. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388998 Free PMC article.
-
Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis.Elife. 2014 Apr 24;3:e01936. doi: 10.7554/eLife.01936. Elife. 2014. PMID: 24843012 Free PMC article.
-
Probing α4βδ GABAA receptor heterogeneity: differential regional effects of a functionally selective α4β1δ/α4β3δ receptor agonist on tonic and phasic inhibition in rat brain.J Neurosci. 2014 Dec 3;34(49):16256-72. doi: 10.1523/JNEUROSCI.1495-14.2014. J Neurosci. 2014. PMID: 25471566 Free PMC article.
-
Traumatic Brain Injury Broadly Affects GABAergic Signaling in Dentate Gyrus Granule Cells.eNeuro. 2021 May 5;8(3):ENEURO.0055-20.2021. doi: 10.1523/ENEURO.0055-20.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33514602 Free PMC article.
References
-
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. α4β3δ GABAA receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem. 2001;276:38934–38939. - PubMed
-
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

