Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media
- PMID: 20176792
- PMCID: PMC2863533
- DOI: 10.1128/IAI.01258-09
Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media
Abstract
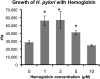
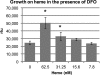
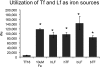
Helicobacter pylori chronically infects the gastric mucosa, where it can be found free in mucus, attached to cells, and intracellularly. H. pylori requires iron for growth, but the sources of iron used in vivo are unclear. In previous studies, the inability to culture H. pylori without serum made it difficult to determine which host iron sources might be used by H. pylori. Using iron-deficient, chemically defined medium, we determined that H. pylori can bind and extract iron from hemoglobin, transferrin, and lactoferrin. H. pylori can use both bovine and human versions of both lactoferrin and transferrin, contrary to previous reports. Unlike other pathogens, H. pylori preferentially binds the iron-free forms of transferrin and lactoferrin, which limits its ability to extract iron from normal serum, which is not iron saturated. This novel strategy may have evolved to permit limited growth in host tissue during persistent colonization while excessive injury or iron depletion is prevented.
Figures
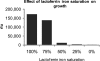

Similar articles
-
Iron acquisition by Helicobacter pylori: importance of human lactoferrin.Infect Immun. 1993 Jun;61(6):2694-7. doi: 10.1128/iai.61.6.2694-2697.1993. Infect Immun. 1993. PMID: 8500909 Free PMC article.
-
Comparison of iron uptake in different Helicobacter species.Res Microbiol. 1999 Sep;150(7):475-81. doi: 10.1016/s0923-2508(99)00109-6. Res Microbiol. 1999. PMID: 10540911
-
Identification, characterization, and immunogenicity of the lactoferrin-binding protein from Helicobacter pylori.Infect Immun. 1997 Feb;65(2):514-8. doi: 10.1128/iai.65.2.514-518.1997. Infect Immun. 1997. PMID: 9009306 Free PMC article.
-
Iron trafficking system in Helicobacter pylori.Biometals. 2012 Apr;25(2):247-58. doi: 10.1007/s10534-011-9512-8. Epub 2011 Nov 30. Biometals. 2012. PMID: 22127376 Review.
-
Bacterial receptors for host transferrin and lactoferrin: molecular mechanisms and role in host-microbe interactions.Future Microbiol. 2013 Dec;8(12):1575-85. doi: 10.2217/fmb.13.125. Future Microbiol. 2013. PMID: 24266357 Review.
Cited by
-
Bacterial chemotaxis in human diseases.Trends Microbiol. 2023 May;31(5):453-467. doi: 10.1016/j.tim.2022.10.007. Epub 2022 Nov 19. Trends Microbiol. 2023. PMID: 36411201 Free PMC article. Review.
-
High resolution electron microscopy of the Helicobacter pylori Cag type IV secretion system pili produced in varying conditions of iron availability.J Vis Exp. 2014 Nov 21;(93):e52122. doi: 10.3791/52122. J Vis Exp. 2014. PMID: 25489938 Free PMC article.
-
Extra-Gastric Manifestations of Helicobacter pylori Infection.J Clin Med. 2020 Nov 30;9(12):3887. doi: 10.3390/jcm9123887. J Clin Med. 2020. PMID: 33265933 Free PMC article. Review.
-
Dietary factors modulate Helicobacter-associated gastric cancer in rodent models.Toxicol Pathol. 2014 Jan;42(1):162-81. doi: 10.1177/0192623313512564. Epub 2013 Dec 3. Toxicol Pathol. 2014. PMID: 24301796 Free PMC article.
-
Helicobacter pylori HP0876 is dispensable for heme-iron acquisition but attenuates bacterial adherence to gastric epithelial cells.Curr Microbiol. 2012 Sep;65(3):254-61. doi: 10.1007/s00284-012-0153-0. Epub 2012 Jun 28. Curr Microbiol. 2012. PMID: 22739662
References
-
- Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. - PubMed
-
- Ascencio, F., A. Ljungh, and T. Wadstrom. 1992. Lactoferrin binding properties of Vibrio cholerae. Microbios 70:103-117. - PubMed
-
- Aspholm, M., F. O. Olfat, J. Norden, B. Sonden, C. Lundberg, R. Sjostrom, S. Altraja, S. Odenbreit, R. Haas, T. Wadstrom, L. Engstrand, C. Semino-Mora, H. Liu, A. Dubois, S. Teneberg, A. Arnqvist, and T. Boren. 2006. SabA is the H. pylori hemagglutinin and is polymorphic in binding to sialylated glycans. PLoS Pathog. 2:e110. - PMC - PubMed
-
- Bahrami, F., A. Elkins, and D. F. Niven. 2003. Iron acquisition by Actinobacillus suis: identification and characterization of transferrin receptor proteins and encoding genes. Vet. Microbiol. 94:79-92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

