Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion
- PMID: 20176801
- PMCID: PMC2839144
- DOI: 10.1084/jem.20090397
Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion
Erratum in
-
Correction: Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion.J Exp Med. 2021 Apr 5;218(4):jem.2009039703042021c. doi: 10.1084/jem.2009039703042021c. J Exp Med. 2021. PMID: 33720318 Free PMC article. No abstract available.
Abstract
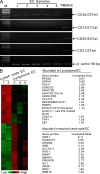
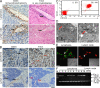
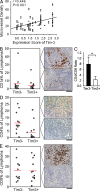
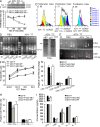
Angiogenesis is increasingly recognized as an important prognosticator associated with the progression of lymphoma and as an attractive target for novel modalities. We report a previously unrecognized mechanism by which lymphoma endothelium facilitates the growth and dissemination of lymphoma by interacting with circulated T cells and suppresses the activation of CD4(+) T cells. Global gene expression profiles of microdissected endothelium from lymphoma and reactive lymph nodes revealed that T cell immunoglobulin and mucin domain-containing molecule 3 (Tim-3) was preferentially expressed in lymphoma-derived endothelial cells (ECs). Clinically, the level of Tim-3 in B cell lymphoma endothelium was closely correlated to both dissemination and poor prognosis. In vitro, Tim-3(+) ECs modulated T cell response to lymphoma surrogate antigens by suppressing activation of CD4(+) T lymphocytes through the activation of the interleukin-6-STAT3 pathway, inhibiting Th1 polarization, and providing protective immunity. In a lymphoma mouse model, Tim-3-expressing ECs promoted the onset, growth, and dissemination of lymphoma by inhibiting activation of CD4(+) T cells and Th1 polarization. Our findings strongly argue that the lymphoma endothelium is not only a vessel system but also a functional barrier facilitating the establishment of lymphoma immune tolerance. These findings highlight a novel molecular mechanism that is a potential target for enhancing the efficacy of tumor immunotherapy and controlling metastatic diseases.
Figures


Similar articles
-
Significance of TIM-3 expression by CD4+ and CD8+ T lymphocytes in tumor-draining lymph nodes from patients with breast cancer.Mol Immunol. 2020 Dec;128:47-54. doi: 10.1016/j.molimm.2020.10.002. Epub 2020 Oct 15. Mol Immunol. 2020. PMID: 33068833
-
Tim-3 expression is increased on peripheral T cells from diffuse large B cell lymphoma.Tumour Biol. 2014 Aug;35(8):7951-6. doi: 10.1007/s13277-014-2080-0. Epub 2014 May 16. Tumour Biol. 2014. PMID: 24833097
-
Genetic Mutations of Tim-3 Ligand and Exhausted Tim-3+ CD8+ T Cells and Survival in Diffuse Large B Cell Lymphoma.J Immunol Res. 2020 Oct 29;2020:6968595. doi: 10.1155/2020/6968595. eCollection 2020. J Immunol Res. 2020. PMID: 33178839 Free PMC article.
-
Emerging immune checkpoints for cancer therapy.Acta Oncol. 2015 Nov;54(10):1706-13. doi: 10.3109/0284186X.2015.1071918. Epub 2015 Sep 11. Acta Oncol. 2015. PMID: 26361073 Review.
-
Regulation of T cell responses by the receptor molecule Tim-3.Immunol Res. 2014 Aug;59(1-3):56-65. doi: 10.1007/s12026-014-8524-1. Immunol Res. 2014. PMID: 24825777 Free PMC article. Review.
Cited by
-
T cell anergy, exhaustion, senescence, and stemness in the tumor microenvironment.Curr Opin Immunol. 2013 Apr;25(2):214-21. doi: 10.1016/j.coi.2012.12.003. Epub 2013 Jan 6. Curr Opin Immunol. 2013. PMID: 23298609 Free PMC article. Review.
-
Expression changes of Tim-3 as one of supplementary indicators for monitoring prognosis of liver pathological changes in chronic HBV infection.BMC Infect Dis. 2022 Nov 11;22(1):842. doi: 10.1186/s12879-022-07841-1. BMC Infect Dis. 2022. PMID: 36368952 Free PMC article.
-
Immunological Regulation of Vascular Inflammation During Cancer Metastasis.Front Immunol. 2019 Aug 21;10:1984. doi: 10.3389/fimmu.2019.01984. eCollection 2019. Front Immunol. 2019. PMID: 31497019 Free PMC article. Review.
-
Tim-3 is highly expressed in T cells in acute myeloid leukemia and associated with clinicopathological prognostic stratification.Int J Clin Exp Pathol. 2014 Sep 15;7(10):6880-8. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25400771 Free PMC article.
-
Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes.Nat Rev Clin Oncol. 2021 Aug;18(8):527-540. doi: 10.1038/s41571-021-00496-y. Epub 2021 Apr 8. Nat Rev Clin Oncol. 2021. PMID: 33833434 Review.
References
-
- Coupel S., Leboeuf F., Boulday G., Soulillou J.P., Charreau B. 2004. RhoA activation mediates phosphatidylinositol 3-kinase-dependent proliferation of human vascular endothelial cells: an alloimmune mechanism of chronic allograft nephropathy. J. Am. Soc. Nephrol. 15:2429–2439 10.1097/01.ASN.0000138237.42675.45 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

