TEX14 interacts with CEP55 to block cell abscission
- PMID: 20176808
- PMCID: PMC2863583
- DOI: 10.1128/MCB.01392-09
TEX14 interacts with CEP55 to block cell abscission
Abstract
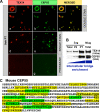
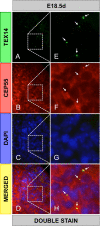
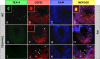
In somatic cells, abscission, the physical separation of daughter cells at the completion of cytokinesis, requires CEP55, ALIX, and TSG101. In contrast, cytokinesis is arrested prior to abscission in differentiating male germ cells that are interconnected by TEX14-positive intercellular bridges. We have previously shown that targeted deletion of TEX14 disrupts intercellular bridges in all germ cells and causes male sterility. Although these findings demonstrate that intercellular bridges are essential for spermatogenesis, it remains to be shown how TEX14 and other proteins come together to prevent abscission and form stable intercellular bridges. Using a biochemical enrichment of male germ cell intercellular bridges, we identified additional bridge proteins, including CEP55. Although CEP55 is highly expressed in testes at the RNA level, there is no report of the presence of CEP55 in germ cells. We show here that CEP55 becomes a stable component of the intercellular bridge and that an evolutionarily conserved GPPX3Y motif of TEX14 binds strongly to CEP55 to block similar GPPX3Y motifs of ALIX and TSG101 from interacting and localizing to the midbody. Thus, TEX14 prevents the completion of cytokinesis by altering the destiny of CEP55 from a nidus for abscission to an integral component of the intercellular bridge.
Figures








Similar articles
-
Structural and biochemical insights into the role of testis-expressed gene 14 (TEX14) in forming the stable intercellular bridges of germ cells.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12372-7. doi: 10.1073/pnas.1418606112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392564 Free PMC article.
-
Inhibition mechanism of testis-expressed gene 14 (TEX14) in cytokinetic abscission: Well-tempered metadynamics simulation studies.J Chem Phys. 2023 Jul 7;159(1):015102. doi: 10.1063/5.0153799. J Chem Phys. 2023. PMID: 37409705
-
Integrin signaling via FAK-Src controls cytokinetic abscission by decelerating PLK1 degradation and subsequent recruitment of CEP55 at the midbody.Oncotarget. 2016 May 24;7(21):30820-30. doi: 10.18632/oncotarget.9003. Oncotarget. 2016. PMID: 27127172 Free PMC article.
-
Germ cell intercellular bridges.Cold Spring Harb Perspect Biol. 2011 Aug 1;3(8):a005850. doi: 10.1101/cshperspect.a005850. Cold Spring Harb Perspect Biol. 2011. PMID: 21669984 Free PMC article. Review.
-
Beyond cytokinesis: the emerging roles of CEP55 in tumorigenesis.Oncogene. 2016 Feb 11;35(6):683-90. doi: 10.1038/onc.2015.128. Epub 2015 Apr 27. Oncogene. 2016. PMID: 25915844 Review.
Cited by
-
Intercellular bridges are essential for transposon repression and meiosis in the male germline.Nat Commun. 2025 Feb 10;16(1):1488. doi: 10.1038/s41467-025-56742-9. Nat Commun. 2025. PMID: 39929837 Free PMC article.
-
Whole exome sequencing in females with autism implicates novel and candidate genes.Int J Mol Sci. 2015 Jan 7;16(1):1312-35. doi: 10.3390/ijms16011312. Int J Mol Sci. 2015. PMID: 25574603 Free PMC article.
-
A simple sperm-sexing method that activates TLR7/8 on X sperm for the efficient production of sexed mouse or cattle embryos.Nat Protoc. 2020 Aug;15(8):2645-2667. doi: 10.1038/s41596-020-0348-y. Epub 2020 Jul 17. Nat Protoc. 2020. PMID: 32681149
-
Structural and biochemical insights into the role of testis-expressed gene 14 (TEX14) in forming the stable intercellular bridges of germ cells.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12372-7. doi: 10.1073/pnas.1418606112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392564 Free PMC article.
-
Actomyosin contractility regulators stabilize the cytoplasmic bridge between the two primordial germ cells during Caenorhabditis elegans embryogenesis.Mol Biol Cell. 2017 Dec 15;28(26):3789-3800. doi: 10.1091/mbc.E17-08-0502. Epub 2017 Oct 26. Mol Biol Cell. 2017. PMID: 29074566 Free PMC article.
References
-
- Braun, R. E., R. R. Behringer, J. J. Peschon, R. L. Brinster, and R. D. Palmiter. 1989. Genetically haploid spermatids are phenotypically diploid. Nature 337:373-376. - PubMed
-
- Carlton, J. G., and J. Martin-Serrano. 2007. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316:1908-1912. - PubMed
-
- Carter, S. L., A. C. Eklund, I. S. Kohane, L. N. Harris, and Z. Szallasi. 2006. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet. 38:1043-1048. - PubMed
-
- Dym, M., and D. W. Fawcett. 1971. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol. Reprod. 4:195-215. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD057880/HD/NICHD NIH HHS/United States
- U54HD07495/HD/NICHD NIH HHS/United States
- T32 HD007495/HD/NICHD NIH HHS/United States
- T32 HD007165/HD/NICHD NIH HHS/United States
- U01 HD060496/HD/NICHD NIH HHS/United States
- U01HD060496/HD/NICHD NIH HHS/United States
- T32GM07330/GM/NIGMS NIH HHS/United States
- U54 HD007495/HD/NICHD NIH HHS/United States
- T32HD007165/HD/NICHD NIH HHS/United States
- R01HD057880/HD/NICHD NIH HHS/United States
- T32 GM007330/GM/NIGMS NIH HHS/United States
- P30 HD007495/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
