A comprehensive model that explains the regulation of phospholipase D2 activity by phosphorylation-dephosphorylation
- PMID: 20176813
- PMCID: PMC2863589
- DOI: 10.1128/MCB.01239-09
A comprehensive model that explains the regulation of phospholipase D2 activity by phosphorylation-dephosphorylation
Abstract
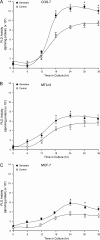
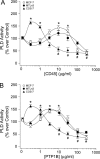
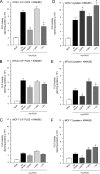
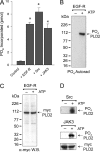
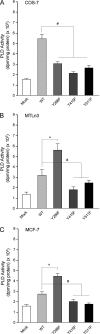
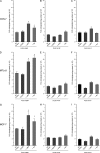
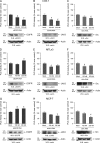
We report here that the enzymatic activity of phospholipase D2 (PLD2) is regulated by phosphorylation-dephosphorylation. Phosphatase treatment of PLD2-overexpressing cells showed a biphasic nature of changes in activity that indicated the existence of "activator" and "inhibitory" sites. We identified three kinases capable of phosphorylating PLD2 in vitro-epidermal growth factor receptor (EGFR), JAK3, and Src (with JAK3 reported for the first time in this study)-that phosphorylate an inhibitory, an activator, and an ambivalent (one that can yield either effect) site, respectively. Mass spectrometry analyses indicated the target of each of these kinases as Y(296) for EGFR, Y(415) for JAK3, and Y(511) for Src. The extent to which each site is activated or inhibited depends on the cell type considered. In COS-7, cells that show the highest level of PLD2 activity, the Y(415) is a prominent site, and JAK3 compensates the negative modulation by EGFR on Y(296). In MCF-7, cells that show the lowest level of PLD2 activity, the converse is the case, with Y(296) unable to compensate the positive modulation by Y(415). MTLn3, with medium to low levels of lipase activity, show an intermediate pattern of regulation but closer to MCF-7 than to COS-7 cells. The negative effect of EGFR on the two cancer cell lines MTLn3 and MCF-7 is further proven by RNA silencing experiments that yield COS-7 showing lower PLD2 activity, and MTLn3 and MCF-7 cells showing an elevated activity. MCF-7 is a cancer cell line derived from a low-aggressive/invasive form of breast cancer that has relatively low levels of PLD activity. We propose that PLD2 activity is low in the breast cancer cell line MCF-7 because it is kept downregulated by tyrosyl phosphorylation of Y(296) by EGFR kinase. Thus, phosphorylation of PLD2-Y(296) could be the signal for lowering the level of PLD2 activity in transformed cells with low invasive capabilities.
Figures

Similar articles
-
The molecular basis of phospholipase D2-induced chemotaxis: elucidation of differential pathways in macrophages and fibroblasts.Mol Cell Biol. 2010 Sep;30(18):4492-506. doi: 10.1128/MCB.00229-10. Epub 2010 Jul 20. Mol Cell Biol. 2010. PMID: 20647543 Free PMC article.
-
Cell invasion of highly metastatic MTLn3 cancer cells is dependent on phospholipase D2 (PLD2) and Janus kinase 3 (JAK3).J Mol Biol. 2011 May 20;408(5):850-62. doi: 10.1016/j.jmb.2011.03.017. Epub 2011 Mar 22. J Mol Biol. 2011. PMID: 21414324 Free PMC article.
-
Serum deprivation confers the MDA-MB-231 breast cancer line with an EGFR/JAK3/PLD2 system that maximizes cancer cell invasion.J Mol Biol. 2013 Feb 22;425(4):755-66. doi: 10.1016/j.jmb.2012.11.035. Epub 2012 Dec 10. J Mol Biol. 2013. PMID: 23238254 Free PMC article.
-
New concepts in phospholipase D signaling in inflammation and cancer.ScientificWorldJournal. 2010 Jul 7;10:1356-69. doi: 10.1100/tsw.2010.116. ScientificWorldJournal. 2010. PMID: 20623096 Free PMC article. Review.
-
The exquisite regulation of PLD2 by a wealth of interacting proteins: S6K, Grb2, Sos, WASp and Rac2 (and a surprise discovery: PLD2 is a GEF).Cell Signal. 2011 Dec;23(12):1885-95. doi: 10.1016/j.cellsig.2011.06.017. Epub 2011 Jun 29. Cell Signal. 2011. PMID: 21740967 Free PMC article. Review.
Cited by
-
Phosphatidic Acid Increases Epidermal Growth Factor Receptor Expression by Stabilizing mRNA Decay and by Inhibiting Lysosomal and Proteasomal Degradation of the Internalized Receptor.Mol Cell Biol. 2015 Sep;35(18):3131-44. doi: 10.1128/MCB.00286-15. Epub 2015 Jun 29. Mol Cell Biol. 2015. PMID: 26124282 Free PMC article.
-
Phospholipase D and phosphatidic acid in the biogenesis and cargo loading of extracellular vesicles.J Lipid Res. 2018 Sep;59(9):1554-1560. doi: 10.1194/jlr.R083964. Epub 2018 May 31. J Lipid Res. 2018. PMID: 29853529 Free PMC article. Review.
-
Phospholipase D inhibitors reduce human prostate cancer cell proliferation and colony formation.Br J Cancer. 2018 Jan;118(2):189-199. doi: 10.1038/bjc.2017.391. Epub 2017 Nov 14. Br J Cancer. 2018. PMID: 29136407 Free PMC article.
-
The molecular basis of phospholipase D2-induced chemotaxis: elucidation of differential pathways in macrophages and fibroblasts.Mol Cell Biol. 2010 Sep;30(18):4492-506. doi: 10.1128/MCB.00229-10. Epub 2010 Jul 20. Mol Cell Biol. 2010. PMID: 20647543 Free PMC article.
-
RGS4 is required for dopaminergic control of striatal LTD and susceptibility to parkinsonian motor deficits.Neuron. 2012 Jan 26;73(2):347-59. doi: 10.1016/j.neuron.2011.11.015. Neuron. 2012. PMID: 22284188 Free PMC article.
References
-
- Aguirre Ghiso, J. A., E. F. Farias, D. F. Alonso, C. Arregui, and E. Bal de Kier Joffe. 1997. A phospholipase D and protein kinase C inhibitor blocks the spreading of murine mammary adenocarcinoma cells altering f-actin and beta1-integrin point contact distribution. Int. J. Cancer 71:881-890. - PubMed
-
- Bae, C. D., D. S. Min, I. N. Fleming, and J. H. Exton. 1998. Determination of interaction sites on the small G protein RhoA for phospholipase D. J. Biol. Chem. 273:11596-11604. - PubMed
-
- Banno, Y., K. Ohguchi, N. Matsumoto, M. Koda, M. Ueda, A. Hara, I. Dikic, and Y. Nozawa. 2005. Implication of phospholipase D2 in oxidant-induced phosphoinositide 3-kinase signaling via Pyk2 activation in PC12 cells. J. Biol. Chem. 280:16319-16324. - PubMed
-
- Brown, H. A., S. Gutowski, C. R. Moomaw, C. Slaughter, and P. C. Sternweis. 1993. ADP-ribosylation factor, a small GTP-dependent regulatory protein, stimulates phospholipase D activity. Cell 75:1137-1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
