Discovery and characterization of inhibitors of Pseudomonas aeruginosa type III secretion
- PMID: 20176902
- PMCID: PMC2863679
- DOI: 10.1128/AAC.01598-09
Discovery and characterization of inhibitors of Pseudomonas aeruginosa type III secretion
Abstract
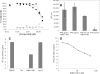
The type III secretion system (T3SS) is a clinically important virulence mechanism in Pseudomonas aeruginosa that secretes and translocates up to four protein toxin effectors into human cells, facilitating the establishment and dissemination of infections. To discover inhibitors of this important virulence mechanism, we developed two cellular reporter assays and applied them to a library of 80,000 compounds. The primary screen was based on the dependence of the transcription of T3SS operons on the T3SS-mediated secretion of a negative regulator and consisted of a transcriptional fusion of the Photorhabdus luminescens luxCDABE operon to the P. aeruginosa exoT effector gene. Secondary assays included direct measurements of the T3SS-mediated secretion of a P. aeruginosa ExoS effector-beta-lactamase fusion protein as well as the detection of the secretion of native ExoS by the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of culture supernatants. Five inhibitors in three chemical classes were demonstrated to inhibit type III secretion selectively with minimal cytotoxicity and with no effects on bacterial growth or on the type II-mediated secretion of elastase. These inhibitors also block the T3SS-mediated secretion of a YopE effector-beta-lactamase fusion protein from an attenuated Yersinia pestis strain. The most promising of the inhibitors is a phenoxyacetamide that also blocks the T3SS-mediated translocation of effectors into mammalian cells in culture. Preliminary studies of structure-activity relationships in this phenoxyacetamide series demonstrated a strict requirement for the R-enantiomer at its stereocenter and indicated tolerance for a variety of substituents on one of its two aromatic rings.
Figures


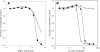
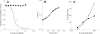
References
-
- Bradley, D. E. 1974. The adsorption of Pseudomonas aeruginosa pilus-dependent bacteriophages to a host mutant with nonretractile pili. Virology 58:149-163. - PubMed
-
- Carpino, L. A. 1993. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J. Am. Chem. Soc. 115:4397-4398.
-
- Choi, K. H., A. Kumar, and H. P. Schweizer. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391-397. - PubMed
-
- Clatworthy, A. E., E. Pierson, and D. T. Hung. 2007. Targeting virulence: a new paradigm for antimicrobial therapy. Nat. Chem. Biol. 3:541-548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

