Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins
- PMID: 20176905
- PMCID: PMC2863644
- DOI: 10.1128/AAC.01432-09
Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins
Abstract
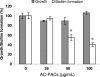
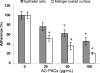
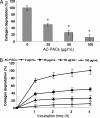
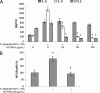
A-type cranberry proanthocyanidins (AC-PACs) have recently been reported to be beneficial for human health, especially urinary tract health. The effect of these proanthocyanidins on periodontitis, a destructive disease of tooth-supporting tissues, needs to be investigated. The purpose of this study was to investigate the effects of AC-PACs on various virulence determinants of Porphyromonas gingivalis as well as on the inflammatory response of oral epithelial cells stimulated by this periodontopathogen. We examined the effects of AC-PACs on P. gingivalis growth and biofilm formation, adherence to human oral epithelial cells and protein-coated surfaces, collagenase activity, and invasiveness. We also tested the ability of AC-PACs to modulate the P. gingivalis-induced inflammatory response by human oral epithelial cells. Our results showed that while AC-PACs neutralized all the virulence properties of P. gingivalis in a dose-dependent fashion, they did not interfere with growth. They also inhibited the secretion of interleukin-8 (IL-8) and chemokine (C-C motif) ligand 5 (CCL5) but did not affect the secretion of IL-6 by epithelial cells stimulated with P. gingivalis. This anti-inflammatory effect was associated with reduced activation of the nuclear factor-kappaB (NF-kappaB) p65 pathway. AC-PACs may be potentially valuable bioactive molecules for the development of new strategies to treat and prevent P. gingivalis-associated periodontal diseases.
Figures
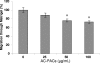
References
-
- Andrian, E., D. Grenier, and M. Rouabhia. 2006. Porphyromonas gingivalis-epithelial cell interactions in periodontitis. J. Dent. Res. 85:392-403. - PubMed
-
- Bavington, C., and C. Page. 2005. Stopping bacterial adhesion: a novel approach to treating infections. Respiration 72:335-344. - PubMed
-
- Bodet, C., F. Chandad, and D. Grenier. 2006. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J. Dent. Res. 85:235-239. - PubMed
-
- Bodet, C., D. Grenier, F. Chandad, I. Ofek, D. Steinberg, and E. I. Weiss. 2008. Potential oral health benefits of cranberry. Crit. Rev. Food Sci. Nutr. 48:672-680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

