Follistatin-like-1, a diffusible mesenchymal factor determines the fate of epithelium
- PMID: 20176958
- PMCID: PMC2842048
- DOI: 10.1073/pnas.0909501107
Follistatin-like-1, a diffusible mesenchymal factor determines the fate of epithelium
Abstract
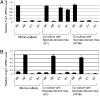
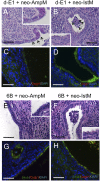
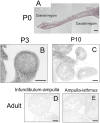
Mesenchyme is generally believed to play critical roles in "secondary induction" during organogenesis. Because of the complexity of tissue interactions in secondary inductions, however, little is known about the precise mechanisms at the cellular and molecular levels. We have demonstrated that, in mouse oviductal development, the mesenchyme determines the fate of undetermined epithelial cells to become secretory or cilial cells. We have established a model for studying secondary induction by establishing clonal epithelial and mesenchymal cell lines from perinatal p53(-/-) mouse oviducts. The signal sequence trap method collected candidate molecules secreted from mesenchymal cell lines. Naive epithelial cells exposed to Follistatin-like-1 (Fstl1), one of the candidates, became irreversibly committed to expressing a cilial epithelial marker and differentiated into ciliated cells. We concluded that Fstl1 is one of the mesenchymal factors determining oviductal epithelial cell fate. This is a unique demonstration that the determination of epithelial cell fate is induced by a single diffusible factor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Mesenchymal signaling in dorsoventral differentiation of palatal epithelium.Cell Tissue Res. 2015 Dec;362(3):541-56. doi: 10.1007/s00441-015-2222-8. Epub 2015 Jun 28. Cell Tissue Res. 2015. PMID: 26123167
-
An evidence of stromal cell populations functionally linked with epithelial cell populations in the mouse oviduct.Zoolog Sci. 2004 Mar;21(3):319-26. doi: 10.2108/zsj.21.319. Zoolog Sci. 2004. PMID: 15056927
-
Fstl1 antagonizes BMP signaling and regulates ureter development.PLoS One. 2012;7(4):e32554. doi: 10.1371/journal.pone.0032554. Epub 2012 Apr 2. PLoS One. 2012. PMID: 22485132 Free PMC article.
-
Mouse oviduct development.Results Probl Cell Differ. 2012;55:247-62. doi: 10.1007/978-3-642-30406-4_14. Results Probl Cell Differ. 2012. PMID: 22918811 Review.
-
The mammalian oviductal epithelium: regional variations in cytological and functional aspects of the oviductal secretory cells.Histol Histopathol. 1996 Jul;11(3):743-68. Histol Histopathol. 1996. PMID: 8839764 Review.
Cited by
-
Biophysical and biochemical properties of Deup1 self-assemblies: a potential driver for deuterosome formation during multiciliogenesis.Biol Open. 2021 Mar 3;10(3):bio056432. doi: 10.1242/bio.056432. Biol Open. 2021. PMID: 33658185 Free PMC article.
-
Follistatin-like protein 1 is a critical mediator of experimental Lyme arthritis and the humoral response to Borrelia burgdorferi infection.Microb Pathog. 2014 Aug;73:70-9. doi: 10.1016/j.micpath.2014.04.005. Epub 2014 Apr 24. Microb Pathog. 2014. PMID: 24768929 Free PMC article.
-
In silico screening system based on a transcription factors regulatory network only using transcriptomic data.PLoS One. 2025 Apr 7;20(4):e0319971. doi: 10.1371/journal.pone.0319971. eCollection 2025. PLoS One. 2025. PMID: 40193394 Free PMC article.
-
Reproductive consequences of developmental phytoestrogen exposure.Reproduction. 2012 Mar;143(3):247-60. doi: 10.1530/REP-11-0369. Epub 2012 Jan 5. Reproduction. 2012. PMID: 22223686 Free PMC article. Review.
-
Follistatin-like 1 in development and human diseases.Cell Mol Life Sci. 2018 Jul;75(13):2339-2354. doi: 10.1007/s00018-018-2805-0. Epub 2018 Mar 29. Cell Mol Life Sci. 2018. PMID: 29594389 Free PMC article. Review.
References
-
- Spemann H. Embryonic development and induction. New Haven, CT: Yale University Press; 1938.
-
- Ruiz i Altaba A. Induction and axial patterning of the neural plate: Planar and vertical signals. J Neurobiol. 1993;24:1276–1304. - PubMed
-
- Grobstein C. Trans-filter induction of tubules in mouse metanephrogenic mesenchyme. Exp Cell Res. 1956;10:424–440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

