Identification of functionally segregated sarcoplasmic reticulum calcium stores in pulmonary arterial smooth muscle
- PMID: 20177054
- PMCID: PMC2859515
- DOI: 10.1074/jbc.M110.101485
Identification of functionally segregated sarcoplasmic reticulum calcium stores in pulmonary arterial smooth muscle
Abstract
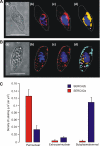
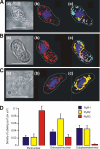
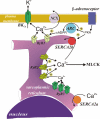
In pulmonary arterial smooth muscle, Ca(2+) release from the sarcoplasmic reticulum (SR) via ryanodine receptors (RyRs) may induce constriction and dilation in a manner that is not mutually exclusive. We show here that the targeting of different sarcoplasmic/endoplasmic reticulum Ca(2+)-ATPases (SERCA) and RyR subtypes to discrete SR regions explains this paradox. Western blots identified protein bands for SERCA2a and SERCA2b, whereas immunofluorescence labeling of isolated pulmonary arterial smooth muscle cells revealed striking differences in the spatial distribution of SERCA2a and SERCA2b and RyR1, RyR2, and RyR3, respectively. Almost all SERCA2a and RyR3 labeling was restricted to a region within 1.5 microm of the nucleus. In marked contrast, SERCA2b labeling was primarily found within 1.5 microm of the plasma membrane, where labeling for RyR1 was maximal. The majority of labeling for RyR2 lay in between these two regions of the cell. Application of the vasoconstrictor endothelin-1 induced global Ca(2+) waves in pulmonary arterial smooth muscle cells, which were markedly attenuated upon depletion of SR Ca(2+) stores by preincubation of cells with the SERCA inhibitor thapsigargin but remained unaffected after preincubation of cells with a second SERCA antagonist, cyclopiazonic acid. We conclude that functionally segregated SR Ca(2+) stores exist within pulmonary arterial smooth muscle cells. One sits proximal to the plasma membrane, receives Ca(2+) via SERCA2b, and likely releases Ca(2+) via RyR1 to mediate vasodilation. The other is located centrally, receives Ca(2+) via SERCA2a, and likely releases Ca(2+) via RyR3 and RyR2 to initiate vasoconstriction.
Figures

Similar articles
-
Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle.Cell Calcium. 2008 Aug;44(2):190-201. doi: 10.1016/j.ceca.2007.11.003. Epub 2008 Jan 11. Cell Calcium. 2008. PMID: 18191199 Free PMC article.
-
Role of InsP3 and ryanodine receptors in the activation of capacitative Ca2+ entry by store depletion or hypoxia in canine pulmonary arterial smooth muscle cells.Br J Pharmacol. 2007 Sep;152(1):101-11. doi: 10.1038/sj.bjp.0707357. Epub 2007 Jun 25. Br J Pharmacol. 2007. PMID: 17592501 Free PMC article.
-
Stretch-induced Ca2+ signalling in vascular smooth muscle cells depends on Ca2+ store segregation.Cardiovasc Res. 2014 Jul 15;103(2):313-23. doi: 10.1093/cvr/cvu069. Epub 2014 Apr 1. Cardiovasc Res. 2014. PMID: 24692174
-
Cross Talk Between Mitochondrial Reactive Oxygen Species and Sarcoplasmic Reticulum Calcium in Pulmonary Arterial Smooth Muscle Cells.Adv Exp Med Biol. 2017;967:289-298. doi: 10.1007/978-3-319-63245-2_17. Adv Exp Med Biol. 2017. PMID: 29047093 Review.
-
The role of intracellular ion channels in regulating cytoplasmic calciumin pulmonary arterial mmooth muscle: which store and where?Adv Exp Med Biol. 2010;661:57-76. doi: 10.1007/978-1-60761-500-2_4. Adv Exp Med Biol. 2010. PMID: 20204723 Review.
Cited by
-
The cell-wide web coordinates cellular processes by directing site-specific Ca2+ flux across cytoplasmic nanocourses.Nat Commun. 2019 May 24;10(1):2299. doi: 10.1038/s41467-019-10055-w. Nat Commun. 2019. PMID: 31127110 Free PMC article.
-
miR-210-5p Promotes Pulmonary Hypertension by Blocking ATP2A2.Cardiovasc Drugs Ther. 2025 Aug;39(4):711-719. doi: 10.1007/s10557-024-07568-y. Epub 2024 Apr 24. Cardiovasc Drugs Ther. 2025. PMID: 38656637
-
Detection of differentially regulated subsarcolemmal calcium signals activated by vasoactive agonists in rat pulmonary artery smooth muscle cells.Am J Physiol Cell Physiol. 2014 Apr 1;306(7):C659-69. doi: 10.1152/ajpcell.00341.2013. Epub 2013 Dec 18. Am J Physiol Cell Physiol. 2014. PMID: 24352334 Free PMC article.
-
Regulation of cellular communication by signaling microdomains in the blood vessel wall.Pharmacol Rev. 2014 Mar 26;66(2):513-69. doi: 10.1124/pr.112.007351. Print 2014. Pharmacol Rev. 2014. PMID: 24671377 Free PMC article. Review.
-
Contractile Behavior of Mouse Aorta Depends on SERCA2 Isoform Distribution: Effects of Replacing SERCA2a by SERCA2b.Front Physiol. 2020 Mar 31;11:282. doi: 10.3389/fphys.2020.00282. eCollection 2020. Front Physiol. 2020. PMID: 32296344 Free PMC article.
References
-
- Nazer M. A., van Breemen C. (1998) Cell Calcium 24, 275–283 - PubMed
-
- van Breemen C., Chen Q., Laher I. (1995) Trends Pharmacol. Sci. 16, 98–105 - PubMed
-
- Iino M., Kobayashi T., Endo M. (1988) Biochem. Biophys. Res. Commun. 152, 417–422 - PubMed
-
- Janiak R., Wilson S. M., Montague S., Hume J. R. (2001) Am. J. Physiol. Cell Physiol. 280, C22–C33 - PubMed
-
- Golovina V. A., Blaustein M. P. (1997) Science 275, 1643–1648 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

