Characterization of the biochemical properties and biological function of the formin homology domains of Drosophila DAAM
- PMID: 20177055
- PMCID: PMC2857102
- DOI: 10.1074/jbc.M109.093914
Characterization of the biochemical properties and biological function of the formin homology domains of Drosophila DAAM
Abstract
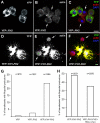
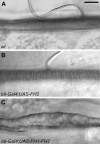
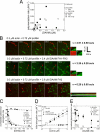
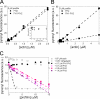
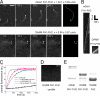
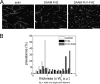
We characterized the properties of Drosophila melanogaster DAAM-FH2 and DAAM-FH1-FH2 fragments and their interactions with actin and profilin by using various biophysical methods and in vivo experiments. The results show that although the DAAM-FH2 fragment does not have any conspicuous effect on actin assembly in vivo, in cells expressing the DAAM-FH1-FH2 fragment, a profilin-dependent increase in the formation of actin structures is observed. The trachea-specific expression of DAAM-FH1-FH2 also induces phenotypic effects, leading to the collapse of the tracheal tube and lethality in the larval stages. In vitro, both DAAM fragments catalyze actin nucleation but severely decrease both the elongation and depolymerization rate of the filaments. Profilin acts as a molecular switch in DAAM function. DAAM-FH1-FH2, remaining bound to barbed ends, drives processive assembly of profilin-actin, whereas DAAM-FH2 forms an abortive complex with barbed ends that does not support profilin-actin assembly. Both DAAM fragments also bind to the sides of the actin filaments and induce actin bundling. These observations show that the D. melanogaster DAAM formin represents an extreme class of barbed end regulators gated by profilin.
Figures


References
-
- Le Clainche C., Carlier M. F. (2008) Physiol. Rev. 88, 489–513 - PubMed
-
- Pollard T. D. (2007) Annu. Rev. Biophys. Biomol. Struct. 36, 451–477 - PubMed
-
- Chhabra E. S., Higgs H. N. (2007) Nat. Cell Biol. 9, 1110–1121 - PubMed
-
- Goode B. L., Eck M. J. (2007) Annu. Rev. Biochem. 76, 593–627 - PubMed
-
- Higgs H. N. (2005) Trends Biochem. Sci. 30, 342–353 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

