A cleavage-resistant urokinase plasminogen activator receptor exhibits dysregulated cell-surface clearance
- PMID: 20177061
- PMCID: PMC2857136
- DOI: 10.1074/jbc.M109.008581
A cleavage-resistant urokinase plasminogen activator receptor exhibits dysregulated cell-surface clearance
Abstract
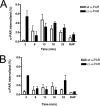
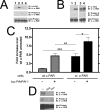
Urokinase plasminogen activator receptor (u-PAR) binds urokinase plasminogen activator (u-PA) and participates in plasminogen activation in addition to modulating several cellular processes such as adhesion, proliferation, and migration. u-PAR is susceptible to proteolysis by its cognate ligand and several other proteases. To elucidate the biological significance of receptor cleavage by u-PA, we engineered and expressed a two-chain urokinase plasminogen activator (tcu-PA) cleavage-resistant u-PAR (cr-u-PAR). This mutated receptor was similar to wild-type u-PAR in binding u-PA and initiating plasminogen activation. However, cr-u-PAR exhibited accelerated internalization and resurfacing due to direct association with the endocytic receptor alpha(2)-macroglobulin receptor/low density lipoprotein receptor-related protein in the absence of the enzyme x inhibitor complex of tcu-PA and plasminogen activator inhibitor-1 (tcu-PA.PAI-1). cr-u-PAR-expressing cells had enhanced migration compared with wild-type u-PAR-expressing cells, and cr-u-PAR was less sensitive to chymotrypsin cleavage as compared with wt u-PAR. Our studies suggest that these mutations in the linker region result in a rearrangement within the cr-u-PAR structure that makes it resemble its ligand-bound form. This constitutively active variant may mimic highly glycosylated cleavage-resistant u-PAR expressed in certain highly malignant cancer-cells.
Figures

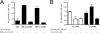
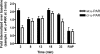


References
-
- Ploug M., Rahbek-Nielsen H., Nielsen P. F., Roepstorff P., Dano K. (1998) J. Biol. Chem. 273, 13933–13943 - PubMed
-
- Blasi F., Stoppelli M. P., Cubellis M. V. (1986) J. Cell. Biochem. 32, 179–186 - PubMed
-
- Møller L. B., Pöllänen J., Rønne E., Pedersen N., Blasi F. (1993) J. Biol. Chem. 268, 11152–11159 - PubMed
-
- Chapman H. A., Wei Y., Simon D. I., Waltz D. A. (1999) Thromb. Haemost. 82, 291–297 - PubMed
-
- Nguyen D. H., Hussaini I. M., Gonias S. L. (1998) J. Biol. Chem. 273, 8502–8507 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

