Human biliverdin reductase suppresses Goodpasture antigen-binding protein (GPBP) kinase activity: the reductase regulates tumor necrosis factor-alpha-NF-kappaB-dependent GPBP expression
- PMID: 20177069
- PMCID: PMC2857060
- DOI: 10.1074/jbc.M109.032771
Human biliverdin reductase suppresses Goodpasture antigen-binding protein (GPBP) kinase activity: the reductase regulates tumor necrosis factor-alpha-NF-kappaB-dependent GPBP expression
Abstract
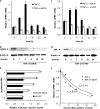
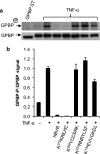
The Ser/Thr/Tyr kinase activity of human biliverdin reductase (hBVR) and the expression of Goodpasture antigen-binding protein (GPBP), a nonconventional Ser/Thr kinase for the type IV collagen of basement membrane, are regulated by tumor necrosis factor (TNF-alpha). The pro-inflammatory cytokine stimulates kinase activity of hBVR and activates NF-kappaB, a transcriptional regulator of GPBP mRNA. Increased GPBP activity is associated with several autoimmune conditions, including Goodpasture syndrome. Here we show that in HEK293A cells hBVR binds to GPBP and down-regulates its TNF-alpha-stimulated kinase activity; this was not due to a decrease in GPBP expression. Findings with small interfering RNA to hBVR and to the p65 regulatory subunit of NF-kappaB show the hBVR role in the initial stimulation of GPBP expression by TNF-alpha-activated NF-kappaB; hBVR was not a factor in mediating GPBP mRNA stability. The interacting domain was mapped to the (281)CX(10)C motif in the C-terminal 24 residues of hBVR. A 7-residue peptide, KKRILHC(281), corresponding to the core of the consensus D(delta)-Box motif in the interacting domain, was as effective as the intact 296-residue hBVR polypeptide in inhibiting GPBP kinase activity. GPBP neither regulated hBVR expression nor TNF-alpha dependent NF-kappaB expression. Collectively, our data reveal that hBVR is a regulator of the TNF-alpha-GPBP-collagen type IV signaling cascade and uncover a novel biological interaction that may be of relevance in autoimmune pathogenesis.
Figures



Similar articles
-
Formation of ternary complex of human biliverdin reductase-protein kinase Cδ-ERK2 protein is essential for ERK2-mediated activation of Elk1 protein, nuclear factor-κB, and inducible nitric-oxidase synthase (iNOS).J Biol Chem. 2012 Jan 6;287(2):1066-79. doi: 10.1074/jbc.M111.279612. Epub 2011 Nov 7. J Biol Chem. 2012. PMID: 22065579 Free PMC article.
-
Characterization of the human biliverdin reductase gene structure and regulatory elements: promoter activity is enhanced by hypoxia and suppressed by TNF-alpha-activated NF-kappaB.FASEB J. 2010 Sep;24(9):3239-54. doi: 10.1096/fj.09-144592. Epub 2010 Apr 21. FASEB J. 2010. PMID: 20410444 Free PMC article.
-
Biliverdin inhibits activation of NF-kappaB: reversal of inhibition by human biliverdin reductase.Int J Cancer. 2007 Dec 1;121(11):2567-74. doi: 10.1002/ijc.22978. Int J Cancer. 2007. PMID: 17683071
-
Potential application of biliverdin reductase and its fragments to modulate insulin/IGF-1/MAPK/PI3-K signaling pathways in therapeutic settings.Curr Drug Targets. 2010 Dec;11(12):1586-94. doi: 10.2174/1389450111009011586. Curr Drug Targets. 2010. PMID: 20704544 Review.
-
Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin.Trends Pharmacol Sci. 2009 Mar;30(3):129-37. doi: 10.1016/j.tips.2008.12.003. Epub 2009 Feb 11. Trends Pharmacol Sci. 2009. PMID: 19217170 Review.
Cited by
-
The human biliverdin reductase-based peptide fragments and biliverdin regulate protein kinase Cδ activity: the peptides are inhibitors or substrate for the protein kinase C.J Biol Chem. 2012 Jul 13;287(29):24698-712. doi: 10.1074/jbc.M111.326504. Epub 2012 May 14. J Biol Chem. 2012. PMID: 22584576 Free PMC article.
-
Formation of ternary complex of human biliverdin reductase-protein kinase Cδ-ERK2 protein is essential for ERK2-mediated activation of Elk1 protein, nuclear factor-κB, and inducible nitric-oxidase synthase (iNOS).J Biol Chem. 2012 Jan 6;287(2):1066-79. doi: 10.1074/jbc.M111.279612. Epub 2011 Nov 7. J Biol Chem. 2012. PMID: 22065579 Free PMC article.
-
GPBP or CERT: The Roles in Autoimmunity, Cancer or Neurodegenerative Disease-A Systematic Review.Int J Mol Sci. 2024 Dec 7;25(23):13179. doi: 10.3390/ijms252313179. Int J Mol Sci. 2024. PMID: 39684889 Free PMC article.
-
Statins more than cholesterol lowering agents in Alzheimer disease: their pleiotropic functions as potential therapeutic targets.Biochem Pharmacol. 2014 Apr 15;88(4):605-16. doi: 10.1016/j.bcp.2013.10.030. Epub 2013 Nov 11. Biochem Pharmacol. 2014. PMID: 24231510 Free PMC article. Review.
-
Goodpasture antigen-binding protein (GPBP) directs myofibril formation: identification of intracellular downstream effector 130-kDa GPBP-interacting protein (GIP130).J Biol Chem. 2011 Oct 7;286(40):35030-43. doi: 10.1074/jbc.M111.249458. Epub 2011 Aug 9. J Biol Chem. 2011. PMID: 21832087 Free PMC article.
References
-
- Hudson B. G., Tryggvason K., Sundaramoorthy M., Neilson E. G. (2003) N. Engl. J. Med. 348, 2543–2556 - PubMed
-
- Saus J. (1998) in Encyclopedia of Immunology, 2nd Ed., pp. 1005–1011, Academic Press, Ltd., London
-
- Granero F., Revert F., Revert-Ros F., Lainez S., Martínez-Martínez P., Saus J. (2005) FEBS J. 272, 5291–5305 - PubMed
-
- Quinones S., Bernal D., García-Sogo M., Elena S. F., Saus J. (1992) J. Biol. Chem. 267, 19780–19784 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

