Heterogeneous microinfarcts caused by coronary microemboli: evaluation with multidetector CT and MR imaging in a swine model
- PMID: 20177087
- PMCID: PMC2826701
- DOI: 10.1148/radiol.09090527
Heterogeneous microinfarcts caused by coronary microemboli: evaluation with multidetector CT and MR imaging in a swine model
Abstract
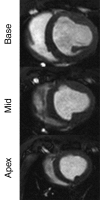
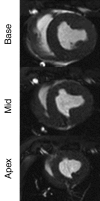
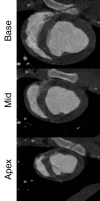
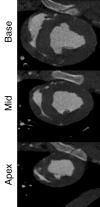
Purpose: To directly compare the sensitivity of 64-section multidetector computed tomography (CT) with that of 1.5-T magnetic resonance (MR) imaging in the depiction and measurement of heterogeneous 7-8-week-old microinfarcts and the quantification of regional left ventricular (LV) function and perfusion in the territory of coronary intervention in a swine model.
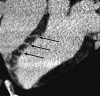
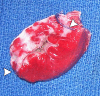
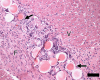
Materials and methods: Approval was obtained from the institutional animal committee. An x-ray/MR system was used to catheterize the left anterior descending (LAD) coronary artery with x-ray guidance and to delineate the perfusion territory. The vessel was selectively microembolized in six pigs with small-diameter embolic material (40-120 microm, 250000 count). At 7-8 weeks after microembolization, multidetector CT and MR imaging were used to assess LV function, first-pass perfusion, and delayed contrast enhancement in remote myocardium and microinfarct scars. Histochemical staining with triphenyltetrazolium chloride (TTC) was used to confirm and quantify heterogeneous microinfarct scars. The two-tailed Wilcoxon signed rank test was used to detect differences between modalities and myocardial regions.
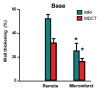
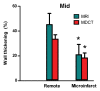
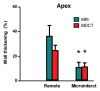
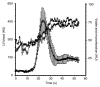
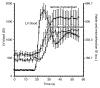
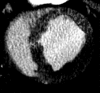
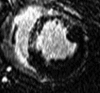
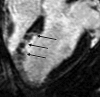
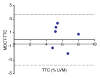
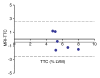
Results: The LAD territory was 32.4% +/- 3.8(stadard error of the mean) of the LV mass. Multidetector CT and MR imaging have similar sensitivity in the detection of regional and global LV dysfunction and extent of microinfarct. The mean LV end-diastolic volume, end-systolic volume, and ejection fraction were 93 mL +/- 8, 46 mL +/- 4, and 50% +/- 3, respectively, on multidetector CT images and 92 mL +/- 8, 48 mL +/- 5, and 48% +/- 3, respectively, on MR images (P > or = .05). The extent of heterogeneous microinfarct was not significantly different between multidetector CT (6.3% +/- 0.8 of the LV mass), MR imaging (6.6% +/- 0.5 of the LV mass), and TTC staining (7.0% +/- 0.6 of the LV mass). First-pass multidetector CT and MR imaging demonstrated significant regional differences (P < .05) in time to peak between the heterogeneous microinfarct and remote myocardium (17.0 seconds +/- 0.3 and 12.4 seconds +/- 0.6, respectively, for multidetector CT and 17.2 seconds +/- 0.8 and 12.5 seconds +/- 1.0, respectively, for MR imaging).
Conclusion: Modern multidetector CT and MR imaging are sensitive modalities with which to depict heterogeneous microinfarcts and determine regional LV dysfunction and decreased perfusion in the territory of intervention. (c) RSNA, 2010.
Figures


Comment in
-
Multidetector CT characterization of microinfarction--is it ready for clinical use?Radiology. 2010 Mar;254(3):637-9. doi: 10.1148/radiol.092323. Radiology. 2010. PMID: 20177078 No abstract available.
References
-
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1–e157 - PubMed
-
- Porto I, Selvanayagam JB, Van Gaal WJ, et al. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation 2006;114:662–669 - PubMed
-
- Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death: autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation 1985;71:699–708 - PubMed
-
- Selvanayagam JB, Cheng AS, Jerosch-Herold M, et al. Effect of distal embolization on myocardial perfusion reserve after percutaneous coronary intervention: a quantitative magnetic resonance perfusion study. Circulation 2007;116:1458–1464 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

