A pertussis toxin sensitive G-protein-independent pathway is involved in serum amyloid A-induced formyl peptide receptor 2-mediated CCL2 production
- PMID: 20177146
- PMCID: PMC2859329
- DOI: 10.3858/emm.2010.42.4.029
A pertussis toxin sensitive G-protein-independent pathway is involved in serum amyloid A-induced formyl peptide receptor 2-mediated CCL2 production
Abstract
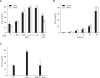
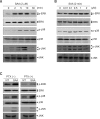
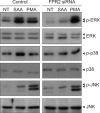
Serum amyloid A (SAA) induced CCL2 production via a pertussis toxin (PTX)-insensitive pathway in human umbilical vein endothelial cells (HUVECs). SAA induced the activation of three MAPKs (ERK, p38 MAPK, and JNK), which were completely inhibited by knock-down of formyl peptide receptor 2 (FPR2). Inhibition of p38 MAPK and JNK by their specific inhibitors (SB203580 and SP600125), or inhibition by a dominant negative mutant of p38 MAPK dramatically decreased SAA-induced CCL2 production. Inactivation of G((i)) protein(s) by PTX inhibited the activation of SAA-induced ERK, but not p38 MAPK or JNK. The results indicate that SAA stimulates FPR2-mediated activation of p38 MAPK and JNK, which are independent of a PTX-sensitive G-protein and are essential for SAA-induced CCL2 production.
Figures
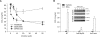
References
-
- Badolato R, Johnston JA, Wang JM, McVicar D, Xu LL, Oppenheim JJ, Kelvin DJ. Serum amyloid A induces calcium mobilization and chemotaxis of human monocytes by activating a pertussis toxin-sensitive signaling pathway. J Immunol. 1995;155:4004–4010. - PubMed
-
- Baranova IN, Vishnyakova TG, Bocharov AV, Kurlander R, Chen Z, Kimelman ML, Remaley AT, Csako G, Thomas F, Eggerman TL, Patterson AP. Serum amyloid A binding to CLA-1 (CD36 and LIMPII analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J Biol Chem. 2005;280:8031–8040. - PubMed
-
- Björkman L, Karlsson J, Karlsson A, Rabiet MJ, Boulay F, Fu H, Bylund J, Dahlgren C. Serum amyloid A mediates human neutrophil production of reactive oxygen species through a receptor independent of formyl peptide receptor like-1. J Leukoc Biol. 2008;83:245–253. - PubMed
-
- Cai L, de Beer MC, de Beer FC, van der Westhuyzen DR. Serum amyloid A is a ligand for scavenger receptor class B type I and inhibits high density lipoprotein binding and selective lipid uptake. J Biol Chem. 2005;280:2954–2961. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
