Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells
- PMID: 20177148
- PMCID: PMC2859327
- DOI: 10.3858/emm.2010.42.4.027
Ovarian cancer-derived lysophosphatidic acid stimulates secretion of VEGF and stromal cell-derived factor-1 alpha from human mesenchymal stem cells
Abstract
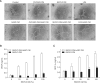
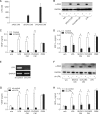
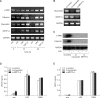
Lysophosphatidic acid (LPA) stimulates growth and invasion of ovarian cancer cells and tumor angiogenesis. Cancer-derived LPA induces differentiation of human adipose tissue-derived mesenchymal stem cells (hASCs) to alpha-smooth muscle actin (alpha-SMA)-positive cancer-associated fibroblasts. Presently, we explored whether cancer-derived LPA regulates secretion of pro-angiogenic factors from hASCs. Conditioned medium (CM) from the OVCAR-3 and SKOV3 ovarian cancer cell lines stimulated secretion angiogenic factors such as stromal-derived factor-1 alpha (SDF-1 alpha) and VEGF from hASCs. Pretreatment with the LPA receptor inhibitor Ki16425 or short hairpin RNA lentiviral silencing of the LPA((1)) receptor abrogated the cancer CM-stimulated expression of alpha-SMA, SDF-1, and VEGF from hASCs. LPA induced expression of myocardin and myocardin-related transcription factor-A, transcription factors involved in smooth muscle differentiation, in hASCs. siRNA-mediated depletion of endogenous myocardin and MRTF-A abrogated the expression of alpha-SMA, but not SDF-1 and VEGF. LPA activated RhoA in hASCs and pretreatment with the Rho kinase inhibitor Y27632 completely abrogated the LPA-induced expression of alpha-SMA, SDF-1, and VEGF in hASCs. Moreover, LPA-induced alpha-SMA expression was abrogated by treatment with the ERK inhibitor U0126 or the phosphoinositide-3-kinase inhibitor LY294002, but not the PLC inhibitor U73122. LPA-induced VEGF secretion was inhibited by LY294002, whereas LPA-induced SDF-1 secretion was markedly attenuated by U0126, U73122, and LY294002. These results suggest that cancer-secreted LPA induces differentiation of hASCs to cancer-associated fibroblasts through multiple signaling pathways involving Rho kinase, ERK, PLC, and phosphoinositide-3-kinase.
Figures



Similar articles
-
Cancer-derived lysophosphatidic acid stimulates differentiation of human mesenchymal stem cells to myofibroblast-like cells.Stem Cells. 2008 Mar;26(3):789-97. doi: 10.1634/stemcells.2007-0742. Epub 2007 Dec 6. Stem Cells. 2008. PMID: 18065393
-
Lysophosphatidic acid-induced ADAM12 expression mediates human adipose tissue-derived mesenchymal stem cell-stimulated tumor growth.Int J Biochem Cell Biol. 2012 Nov;44(11):2069-76. doi: 10.1016/j.biocel.2012.08.004. Epub 2012 Aug 10. Int J Biochem Cell Biol. 2012. PMID: 22903068
-
Proteomic identification of betaig-h3 as a lysophosphatidic acid-induced secreted protein of human mesenchymal stem cells: paracrine activation of A549 lung adenocarcinoma cells by betaig-h3.Mol Cell Proteomics. 2012 Feb;11(2):M111.012385. doi: 10.1074/mcp.M111.012385. Epub 2011 Dec 7. Mol Cell Proteomics. 2012. PMID: 22159598 Free PMC article.
-
Lysophosphatidic Acid Regulates Rho Family of GTPases in Lungs.Cell Biochem Biophys. 2021 Sep;79(3):493-496. doi: 10.1007/s12013-021-00993-y. Epub 2021 Jun 10. Cell Biochem Biophys. 2021. PMID: 34110567 Review.
-
Colorectal cancer cells - Proliferation, survival and invasion by lysophosphatidic acid.Int J Biochem Cell Biol. 2010 Dec;42(12):1907-10. doi: 10.1016/j.biocel.2010.09.021. Int J Biochem Cell Biol. 2010. PMID: 20932934 Free PMC article. Review.
Cited by
-
How Autophagy Shapes the Tumor Microenvironment in Ovarian Cancer.Front Oncol. 2020 Dec 7;10:599915. doi: 10.3389/fonc.2020.599915. eCollection 2020. Front Oncol. 2020. PMID: 33364196 Free PMC article. Review.
-
CARMA3: A novel scaffold protein in regulation of NF-κB activation and diseases.World J Biol Chem. 2010 Dec 26;1(12):353-61. doi: 10.4331/wjbc.v1.i12.353. World J Biol Chem. 2010. PMID: 21537470 Free PMC article.
-
Mesenchymal stem cells in tissue repair.Front Immunol. 2013 Sep 4;4:201. doi: 10.3389/fimmu.2013.00201. Front Immunol. 2013. PMID: 24027567 Free PMC article. Review.
-
The Dual Role of Mesenchymal Stem Cells in Cancer Pathophysiology: Pro-Tumorigenic Effects versus Therapeutic Potential.Int J Mol Sci. 2023 Aug 31;24(17):13511. doi: 10.3390/ijms241713511. Int J Mol Sci. 2023. PMID: 37686315 Free PMC article. Review.
-
Optimizing molecular-targeted therapies in ovarian cancer: the renewed surge of interest in ovarian cancer biomarkers and cell signaling pathways.J Oncol. 2012;2012:737981. doi: 10.1155/2012/737981. Epub 2012 Feb 6. J Oncol. 2012. PMID: 22481932 Free PMC article.
References
-
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
-
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–465. - PubMed
-
- Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–489. - PubMed
-
- Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. - PubMed
-
- Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A, Saffrich R, Schubert M, Ho AD, Giese N, Buchler MW, Friess H, Buchler P, Herr I. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–631. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
