Co-treatment with hepatocyte growth factor and TGF-beta1 enhances migration of HaCaT cells through NADPH oxidase-dependent ROS generation
- PMID: 20177149
- PMCID: PMC2859326
- DOI: 10.3858/emm.2010.42.4.026
Co-treatment with hepatocyte growth factor and TGF-beta1 enhances migration of HaCaT cells through NADPH oxidase-dependent ROS generation
Abstract
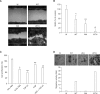
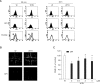
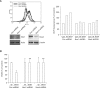
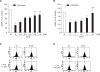
Wound healing requires re-epithelialization from the wound margin through keratinocyte proliferation and migration, and some growth factors are known to influence this process. In the present study, we found that the co-treatment with hepatocyte growth factor (HGF) and TGF-beta1 resulted in enhanced migration of HaCaT cells compared with either growth factor alone, and that N-acetylcysteine, an antioxidant agent, was the most effective among several inhibitors tested, suggesting the involvement of reactive oxygen species (ROS). Fluorescence-activated cell sorter analysis using 2,7-dichlorofluorescein diacetate (DCF-DA) dye showed an early (30 min) as well as a late (24 h) increase of ROS after scratch, and the increase was more prominent with the growth factor treatment. Diphenyliodonium (DPI), a potent inhibitor of NADPH oxidase, abolished the increase of ROS at 30 min, followed by the inhibition of migration, but not the late time event. More precisely, gene knockdown by shRNA for either Nox-1 or Nox-4 isozyme of gp91phox subunit of NADPH oxidase abolished both the early time ROS production and migration. However, HaCaT cell migration was not enhanced by treatment with H((2))O((2)). Collectively, co-treatment with HGF and TGF-beta1 enhances keratinocyte migration, accompanied with ROS generation through NADPH oxidase, involving Nox-1 and Nox-4 isozymes.
Figures
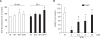
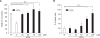
Similar articles
-
TGF-beta-induced p38 activation is mediated by Rac1-regulated generation of reactive oxygen species in cultured human keratinocytes.Int J Mol Med. 2001 Sep;8(3):251-5. Int J Mol Med. 2001. PMID: 11494050
-
Role of reactive oxygen species in transforming growth factor beta1-induced alpha smooth-muscle actin and collagen production in nasal polyp-derived fibroblasts.Int Arch Allergy Immunol. 2012;159(3):278-86. doi: 10.1159/000337460. Epub 2012 Jun 21. Int Arch Allergy Immunol. 2012. PMID: 22722757
-
Oxidative stress in scleroderma: maintenance of scleroderma fibroblast phenotype by the constitutive up-regulation of reactive oxygen species generation through the NADPH oxidase complex pathway.Arthritis Rheum. 2001 Nov;44(11):2653-64. doi: 10.1002/1529-0131(200111)44:11<2653::aid-art445>3.0.co;2-1. Arthritis Rheum. 2001. PMID: 11710721
-
Nox proteins in signal transduction.Free Radic Biol Med. 2009 Nov 1;47(9):1239-53. doi: 10.1016/j.freeradbiomed.2009.07.023. Epub 2009 Jul 21. Free Radic Biol Med. 2009. PMID: 19628035 Free PMC article. Review.
-
Downstream targets and intracellular compartmentalization in Nox signaling.Antioxid Redox Signal. 2009 Oct;11(10):2467-80. doi: 10.1089/ars.2009.2594. Antioxid Redox Signal. 2009. PMID: 19309256 Free PMC article. Review.
Cited by
-
Retracted Article: MiR-132 enhances proliferation and migration of HaCaT cells by targeting TIMP3.RSC Adv. 2019 Jul 5;9(37):21125-21133. doi: 10.1039/c8ra10552a. eCollection 2019 Jul 5. RSC Adv. 2019. Retraction in: RSC Adv. 2021 Jan 26;11(9):5002. doi: 10.1039/d1ra90046c. PMID: 35521312 Free PMC article. Retracted.
-
NADPH oxidase NOX4 supports renal tumorigenesis by promoting the expression and nuclear accumulation of HIF2α.Cancer Res. 2014 Jul 1;74(13):3501-3511. doi: 10.1158/0008-5472.CAN-13-2979. Epub 2014 Apr 22. Cancer Res. 2014. PMID: 24755467 Free PMC article.
-
TGFβ-induced cytoskeletal remodeling mediates elevation of cell stiffness and invasiveness in NSCLC.Sci Rep. 2019 May 21;9(1):7667. doi: 10.1038/s41598-019-43409-x. Sci Rep. 2019. PMID: 31113982 Free PMC article.
-
NADPH oxidase 1 and its derived reactive oxygen species mediated tissue injury and repair.Oxid Med Cell Longev. 2014;2014:282854. doi: 10.1155/2014/282854. Epub 2014 Jan 19. Oxid Med Cell Longev. 2014. PMID: 24669283 Free PMC article. Review.
-
Hepatocyte growth factor-loaded biomaterials for mesenchymal stem cell recruitment.Stem Cells Int. 2013;2013:892065. doi: 10.1155/2013/892065. Epub 2013 Jun 18. Stem Cells Int. 2013. PMID: 23861688 Free PMC article.
References
-
- Ago T, Nunoi H, Ito T, Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox). Triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem. 1999;274:33644–33653. - PubMed
-
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. - PubMed
-
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem. 1997;272:217–221. - PubMed
-
- Bae YS, Sung JY, Kim OS, Kim YJ, Hur KC, Kazlauskas A, Rhee SG. Platelet-derived growth factor-induced H(2)O(2) production requires the activation of phosphatidylinositol 3-kinase. J Biol Chem. 2000;275:10527–10531. - PubMed
-
- Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–C1224. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
