Spectroscopic and electrochemical analysis of psychotropic drugs
- PMID: 20177449
- PMCID: PMC2810060
- DOI: 10.4103/0250-474X.51942
Spectroscopic and electrochemical analysis of psychotropic drugs
Abstract
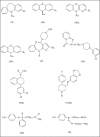
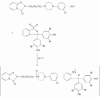
Psychotropic drugs are an important family of compounds from a medical point of view. Their application in therapy requires methods for the determination in pharmaceutical dosage forms and body fluids. Several methods for their analysis have been reported in the literature. Among the methods, spectrophotometric and electrochemical are very useful for the determination of the drugs. Some of the spectrophotometric methods are based on the formation of the binary and ternary compounds with complexes of metals. The formed compounds are sparingly soluble in water, but quantitatively extracted from aqueous phase into organic solvents and the extracts are intensely colored and stable for a few days. These complexes have been employed in pharmaceutical analysis. The electrochemical procedures are very useful in determination of the psychotropic substances in pharmaceutical preparations.
Keywords: Psychotropic drugs; analysis; spectrophotometric and electrochemical methods.
Figures


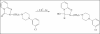
Similar articles
-
High perfomance liquid chromatography in pharmaceutical analyses.Bosn J Basic Med Sci. 2004 May;4(2):5-9. doi: 10.17305/bjbms.2004.3405. Bosn J Basic Med Sci. 2004. PMID: 15629016 Free PMC article.
-
Spectrophotometric determination of benzydamine HCl, levamisole HCl and mebeverine HCl through ion-pair complex formation with methyl orange.Spectrochim Acta A Mol Biomol Spectrosc. 2008 Mar;69(3):770-5. doi: 10.1016/j.saa.2007.04.032. Epub 2007 May 21. Spectrochim Acta A Mol Biomol Spectrosc. 2008. PMID: 17625955
-
Extractive-spectrophotometric determination of some phenothiazines with dipicrylamine and picric acid.J Pharm Biomed Anal. 2002 Jan 1;27(1-2):335-40. doi: 10.1016/s0731-7085(01)00520-9. J Pharm Biomed Anal. 2002. PMID: 11682241
-
Application of phenothiazine derivatives and other compounds for the determination of metals in various samples.Curr Drug Targets. 2006 Sep;7(9):1107-21. doi: 10.2174/138945006778226688. Curr Drug Targets. 2006. PMID: 17017889 Review.
-
[Crystalline molecular complexes generated between surfactants and various additive compounds: an attempt to modify sparingly water soluble drugs to easily water soluble drugs].Yakugaku Zasshi. 2001 Jun;121(6):403-21. doi: 10.1248/yakushi.121.403. Yakugaku Zasshi. 2001. PMID: 11433775 Review. Japanese.
Cited by
-
Density functional theory study of structural and electronic properties of trans and cis structures of thiothixene as a nano-drug.J Mol Model. 2017 Nov 25;23(12):356. doi: 10.1007/s00894-017-3522-6. J Mol Model. 2017. PMID: 29177682
References
-
- GuptaR RR, editor. Phenothiazines and 1,4-Benzothiazines. Vol. IV. Elsevier: Analytical Application of Phenothiazines, Amsterdam; 1998. Bioactive Molecules; pp. 861–98. Chapter XVI.
-
- Kojło A, Karpińska J, Kuźmicka L, Misiuk W, Puzanowska-Tarasiewicz H, Tarasiewicz M. Analytical study of the reaction of phenothiazines with some oxidants, metal ions, and organic substances. J Trace Microprobe Tech. 2001;19:45–70.
-
- Puzanowska-Tarasiewicz H, Tarasiewicz M, Karpińska J, Kojło A, Wołyniec E, Kleszczewska E. Analytical application of the reactions of 2- and 10-disubstituted phenothiazines with some oxidizing agents. Chem Anal (Warsaw) 1998;43:159–78.
-
- Tarasiewicz M, Puzanowska-Tarasiewicz H, Misiuk W, Kojło A, Grudniewska A, Starczewska B. Analytical applications of the reactions of 2- and 10-disubstituted phenothiazines with some metal ions. Chem Anal (Warsaw) 1999;44:137–55.
-
- Karpińska J, Starczewska B, Puzanowska-Tarasiewicz H. Analytical properties of 2- and 10-disubstituted phenothiazines derivatives. Anal Sci. 1996;12:161–70.
LinkOut - more resources
Full Text Sources
