Bioorthogonal chemistry: recent progress and future directions
- PMID: 20177591
- PMCID: PMC2914230
- DOI: 10.1039/b925931g
Bioorthogonal chemistry: recent progress and future directions
Abstract
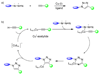
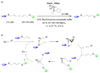
The ability to use covalent chemistry to label biomolecules selectively in their native habitats has greatly enhanced our understanding of biomolecular dynamics and function beyond what is possible with genetic tools alone. To attain the exquisite selectivity that is essential in this covalent approach a "bottom-up" two-step strategy has achieved many successes recently. In this approach, a bioorthogonal chemical functionality is built into life's basic building blocks-amino acids, nucleosides, lipids, and sugars-as well as coenzymes; after the incorporation, an array of biophysical probes are selectively appended to the tagged biomolecules via a suitable bioorthogonal reaction. While much has been accomplished in the expansion of non-natural building blocks carrying unique chemical moieties, the dearth of robust bioorthogonal reactions has limited both the scope and utility of this promising approach. Here, we summarize the recent progress in the development of bioorthogonal reactions and their applications in various biological systems. A major emphasis has been placed on the mechanistic and kinetic studies of these reactions with the hope that continuous improvements can be made with each reaction in the future. In view of the gap between the capabilities of the current repertoire of bioorthogonal reactions and the unmet needs of outstanding biological problems, we also strive to project the future directions of this rapidly developing field.
Figures
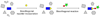







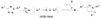


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

