Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols
- PMID: 20177770
- PMCID: PMC2854988
- DOI: 10.1007/s10815-010-9394-7
Qualitative and morphometric analysis of the ultrastructure of human oocytes cryopreserved by two alternative slow cooling protocols
Abstract
Purpose: To ascertain possible cell damage from cryopreservation, the ultrastructure of human oocytes cryopreserved by slow cooling was assessed.
Materials and methods: Cryopreservation was performed through two protocols with one-step or two-step propanediol. Fresh control oocytes were examined for comparison. Samples were processed for transmission electron microscopy analysis.
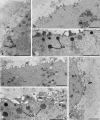
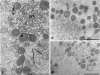
Results: By light microscopy, both fresh and frozen-thawed oocytes appeared regularly rounded, with intact zona pellucida, and homogeneous cytoplasm. By electron microscopy observation, organelles were abundant and uniformly dispersed. Mitochondria-smooth endoplasmic reticulum associations appeared regular. However, both the amount and density of cortical granules appeared abnormally reduced in frozen-thawed samples. Slight to moderate vacuolization was also found in the ooplasm of oocytes of both frozen groups.
Conclusions: Slow cooling ensures a good overall preservation of human oocytes. However, cytoplasmic vacuolization and cortical granule loss appears associated with cryopreservation, irrespective of the protocol used.
Figures


References
-
- Al-Hasani S, Diedrich K, Ven H, Reinecke A, Hartje M, Krebs D. Cryopreservation of human oocytes. Hum Reprod. 1987;2:695–700. - PubMed
-
- Bianchi V, Coticchio G, Distratis V, Giusto N, Flamigni C, Borini A. Differential sucrose concentration during dehydration (0.2 mol/l) and rehydration (0.3 mol/l) increases the implantation rate of frozen human oocytes. Reprod Biomed Online. 2007;14:64–71. - PubMed
-
- Stachecki JJ, Cohen J, Garrisi J, Munne S, Burgess C, Willadsen SM. Cryopreservation of unfertilized human oocytes. Reprod Biomed Online. 2006;13:222–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

