The effect of duodenal-jejunal bypass on glucose-dependent insulinotropic polypeptide secretion in Wistar rats
- PMID: 20177809
- PMCID: PMC3761799
- DOI: 10.1007/s11695-010-0095-1
The effect of duodenal-jejunal bypass on glucose-dependent insulinotropic polypeptide secretion in Wistar rats
Abstract
Background: Enteroendocrine K cells secrete the incretin hormone glucose-dependent insulinotropic peptide (GIP) and are predominately located in the duodenum. GIP levels should decrease after gastric bypass due to duodenal exclusion; however, studies have found conflicting data regarding the changes in GIP secretion after gastric bypass and duodenal-jejunal bypass (DJB).
Methods: We performed a DJB or Sham surgery on Wistar rats followed by an oral glucose tolerance test on postoperative (post-op) day 12 and superior mesenteric lymphatic cannulation on post-op day 14. We measured meal-stimulated GIP concentrations and small bowel GIP and GLP-1 protein content after DJB or Sham surgery.
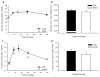
Results: There was no difference in glucose tolerance by 12 days post-op. We found no difference in lymphatic GIP concentration area under the curve between DJB and Sham rats (15,240 pg/ml min +/- 2,651 vs. 17,201 pg/ml min +/- 2,763, respectively, p = 0.62). GIP and GLP-1 protein contents were both significantly increased only in the midjejunum in DJB rats compared to Sham rats (p = 0.009 and p = 0.01, respectively).
Conclusions: Plasma and lymphatic GIP concentrations did not significantly change after DJB in Wistar rats. DJB increased GIP protein content in the midjejunum at the new site of nutrient absorption, but this was surprisingly not countered by a decrease in GIP protein content in the bypassed duodenum. Further studies are needed to determine the mechanisms that account for the discrepancy in GIP production and subsequent secretion after DJB as well as what role GIP plays in the effect of gastrointestinal surgery on glucose homeostasis.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
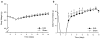
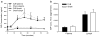
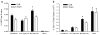
Similar articles
-
Duodenal-jejunal bypass protects GK rats from {beta}-cell loss and aggravation of hyperglycemia and increases enteroendocrine cells coexpressing GIP and GLP-1.Am J Physiol Endocrinol Metab. 2011 May;300(5):E923-32. doi: 10.1152/ajpendo.00422.2010. Epub 2011 Feb 8. Am J Physiol Endocrinol Metab. 2011. PMID: 21304061
-
Duodenal-jejunal bypass and jejunectomy improve insulin sensitivity in Goto-Kakizaki diabetic rats without changes in incretins or insulin secretion.Diabetes. 2014 Mar;63(3):1069-78. doi: 10.2337/db13-0856. Epub 2013 Nov 15. Diabetes. 2014. PMID: 24241532
-
Influence of New Modified Biliopancreatic Diversion on Blood Glucose and Lipids in GK rats.Obes Surg. 2017 Mar;27(3):657-664. doi: 10.1007/s11695-016-2320-z. Obes Surg. 2017. PMID: 27525641
-
Glucose-dependent insulinotropic polypeptide: effects on insulin and glucagon secretion in humans.Dan Med J. 2016 Apr;63(4):B5230. Dan Med J. 2016. PMID: 27034187 Review.
-
[Glucose-dependent insulinotropic peptide in Type 2 diabetes after gastric bypass surgery].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011 Oct;36(10):1017-20. doi: 10.3969/j.issn.1672-7347.2011.10.015. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011. PMID: 22086011 Review. Chinese.
Cited by
-
Duodenal Exclusion but Not Sleeve Gastrectomy Preserves Insulin Secretion, Making It the More Effective Metabolic Procedure.Obes Surg. 2018 May;28(5):1408-1416. doi: 10.1007/s11695-017-3045-3. Obes Surg. 2018. PMID: 29235009
-
Bypassing the duodenum does not improve insulin resistance associated with diet-induced obesity in rodents.Obesity (Silver Spring). 2011 Feb;19(2):380-7. doi: 10.1038/oby.2010.263. Epub 2010 Oct 28. Obesity (Silver Spring). 2011. PMID: 21030948 Free PMC article.
-
Differential Impact of Medical Therapies for Acromegaly on Glucose Metabolism.Int J Mol Sci. 2025 Jan 8;26(2):465. doi: 10.3390/ijms26020465. Int J Mol Sci. 2025. PMID: 39859181 Free PMC article. Review.
-
GIP and bariatric surgery.Obes Surg. 2011 Feb;21(2):244-52. doi: 10.1007/s11695-010-0305-x. Obes Surg. 2011. PMID: 21082290 Review.
-
Effects of duodeno-jejunal bypass on glucose metabolism in obese rats with type 2 diabetes.Surg Today. 2014 Feb;44(2):340-8. doi: 10.1007/s00595-013-0638-x. Epub 2013 Jun 20. Surg Today. 2014. PMID: 23784107
References
-
- Sjöström CD, Lissner L, Wedel H, et al. Reduction in incidence of diabetes, hypertension, and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–84. - PubMed
-
- Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. NEJM. 2004;351:2683–93. - PubMed
-
- Nauck MA, Bartels E, Orskov C, et al. Additive insulinotropic effects of exogenous synthetic human gastric inhibitory polypeptide and glucagon-like peptide-1-(7-36) amide infused at near-physiological insulinotropic hormone and glucose concentrations. J Clin Endocrinol Metab. 1993;76:912–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

